Abstract
Background
To estimate the diagnostic utility of chest CT qualitative assessment and chest CT total severity score (TSS) to predict mortality in oncology patients with COVID-19 infection.
Methods
This retrospective study included 151 oncology patients with COVID-19 infection. 67, 84 were male and female, respectively. Their mean age (years) ± SD was 49.7 ± 14.9. Two radiologists individually reviewed the chest CT and scored the pulmonary abnormalities using TSS. Inter-observer agreement was determined using the Bland–Altman plot. Correlation between TSS and COVID-19 severity, complication, mortality, cancer status and effect in anticancer therapy plan was done.
Results
There was a statistically significant excellent agreement between the independent observers in quantitative pulmonary assessment using TSS with interclass correlation (ICC) > 0.9 (P < 0.001). ROC curve analysis revealed that TSS was statistically significantly higher in non-survivors using an optimum cut-off value of 5 to predict in-hospital mortality. Univariate analysis showed that age, pulmonary predominant pattern, pleural effusion, tree-in-bud, ECOG PS, tumour stage 4 and post-COVID cancer status were a statistically significant predictor of mortality. Multivariate analysis reported that consolidation versus ground-glass opacity (GGO), crazy paving pattern versus GGO and progressive versus remittent cancer diseases were statistically significant independent predictors of mortality among those patients.
Conclusions
TSS demonstrated excellent inter-observer agreement to assess COVID-19 in oncology patients with low cut-off value to predict in-hospital mortality, thus raising the attention to rapid proper care in this setting. There was a statistically significant positive correlation between TSS and delayed chemotherapeutic schedule.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Key points
-
TSS revealed an excellent inter-observer agreement to assess in-hospital mortality in oncology patients with COVID-19 infection.
-
TSS revealed a lower cut-off to predict in-hospital mortality in oncology patients compared to the general population. There was a statistically significant positive correlation between TSS and delay time to resume chemotherapy as well as post-COVID-19 cancer status.
-
Predominant pulmonary pattern of consolidation or crazy paving pattern versus GGO and progressive diseases versus remittent diseases were statistically significant independent predictors of mortality among oncology patients with COVID-19 infection.
Background
Coronavirus disease 2019 (COVID-19) first appeared in Wuhan, China, in December 2019, then rapidly spread causing an outbreak [1]. The immunocompromised status of oncology patients (due to the disease itself or the treatment) raises their risk of infection [2]. Oncology patients deteriorate more rapidly with higher mortality than the general population with COVID-19 infection [3]. COVID-19 infection must be excluded preceding admission of oncology patients for anticancer therapies, also postponing those therapies should be considered according to patient performance status [4].
Clinical presentation of COVID-19 ranges from mild symptoms to severe disease such as acute respiratory distress syndrome necessitating mechanical ventilation and multi-organ failure [5]. Oncology patients had a higher mortality rate and intensive care unit (ICU) admissions as compared to non-cancer patients; therefore, early detection of COVID-19 can improve their management [6]. Also, oncology patients may experience long-term COVID-19 sequelae [7].
The reverse transcription–polymerase chain reaction assay (RT-PCR) assesses viral load and is considered the standard diagnostic means of COVID-19 infection [8]. Chest CT is a critical component in the diagnostic algorithm for patients with suspected COVID-19 infection [9]. Even few studies considered that chest CT is a more rapid, sensitive and effective tool than RT-PCR [10, 11]. The National Health Commission of the People’s Republic of China has promoted diagnosis based on clinical and chest CT findings alone due to the shortage of PCR kits in extreme situations and the possibility of false negative RT-PCR results [12]. Previous studies reported low sensitivity of RT-PCR in the early presentation, while chest CT has established a higher sensitivity in detecting COVID-19 (37–71% and 56–98%, respectively) [13].
The CT findings of COVID-19 infection most frequently comprise multiple peripheral, ground-glass opacities (GGO) which may be accompanied by consolidation [14], also comprising a crazy paving pattern, and bronchial wall thickening [15]. CT is also particularly critical in oncology patients for the differential diagnosis of drug-induced pneumonitis and cancer progression [16].
Variable chest CT grading systems have been suggested to evaluate the severity of pulmonary involvement in COVID-19 infection [9, 17, 18]. A recent comparative study investigated the diagnostic performance of different CT scoring systems and concluded that the CT total severity score (TSS) had the highest specificity and the least time consumption [19]. Also, a recent study focused on selected high-risk groups with renal impairment considered high TSS as a substantial predictor of mortality [20].
To the best of our knowledge, the studies on quantitative chest CT evaluation of COVID-19 infection in oncology patients are limited, till now no available studies discussed the performance of TSS.
Therefore, we aimed to investigate the diagnostic utility and inter-reader agreement of the TSS for predicting the survival, morbidity and effect on treatment plans among oncology patients after COVID-19 infection.
Methods
Study population
This is a retrospective single-centre study conducted on oncology patients with COVID-19 infection who underwent chest CT from June 2020 to May 2022. The study was approved by the Institutional Review Board of our university (Approval No: R.22.06.1738) and a waiver of the consent of medical record review was received. One hundred seventy patients were initially enrolled in this study. Then, 19 patients were excluded: two patients with negative findings at chest CT, eight patients with unavailable CT images upon admission and nine patients with incomplete clinical data. Lastly, 151 consecutive patients were included, the flowchart is demonstrated in Additional file 1: Fig. S1. 67, 84 were male and female, respectively. Their mean age (years) ± SD was 49.7 ± 14.9 ranging from 19 to 82 years.
Clinical data
Clinical data involved the duration of symptoms of COVID-19 infection, type of malignancy and its stage, type of anticancer therapy, Eastern Cooperative Oncology Group (ECOG) performance status, duration between the last chemotherapy and COVID-19 diagnosis and duration to resume chemotherapy. Laboratory data mainly included PCR results, arterial blood gases, serum creatinine and liver function tests. The need for oxygen supply, steroids, mechanical ventilation and ICU admission was reported.
Chest CT acquisition
All patients underwent chest CT imaging on a 16-detector CT scanner (Bright speed; GE Healthcare). Images were attained during a single inspiratory breath-hold. CT scan parameters were as follows: X-ray tube parameters, 120 KVp, 350mAs; rotation time, 0.5 s; pitch, 1.0; section thickness, 5 mm; intersection space, 5 mm; additional reconstruction with a slice thickness of 1.5 mm. Scans were reviewed at a window width and level of 1000 to 2000 HU and − 700 to − 500 HU, respectively, to assess the lung parenchyma.
Qualitative CT image analysis
All chest CT images obtained at the time of hospital admission were analysed. The main findings were described as the following: GGO, consolidation and crazy paving pattern based on the standard lexicon for thoracic imaging reported by the Fleischner Society and similar previous studies [21]. The predominant pattern, presence of pleural effusion, pulmonary nodules, tree-in-bud pattern, bronchial dilatation and mediastinal lymphadenopathy were reported.
Quantitative CT image analysis
The severity of pulmonary parenchymal involvement was quantified by the TSS. TSS is a quantitative score considering the pulmonary abnormalities in each of the five lobes of both lungs, involving the existence of GGOs, consolidation or mixed GGO [22]. All CT images were independently analysed by two radiologists (13 and 10 years of experience in the interpretation of chest CT scans). Both radiologists were blinded to clinical data. According to the extent of pulmonary involvement, each lobe could be scored from 0 to 4 points as the following: 0, no involvement; 1, from 1 to 25% involvement;2, from 26 to 50% involvement; 3, from 51 to75% involvement; and 4, more than 75% involvement. The sum of each lobar score resulted in the TSS which ranged from 0 to 20.
Statistical analysis
Data were entered and analysed using IBM SPSS Statistics for Windows (version 26). IBM Corp. Released 2019., Armonk, NY. Qualitative data were expressed as frequency (N) and percentage (%). The intraclass correlation coefficient (ICC) was used to determine inter-observer reliability (both consistency and absolute agreement). The Bland–Altman plot was used to compare TSS measurements by plotting the differences against the averages of the two observers. Spearman’s correlation was used to assess the relationship between TSS score and cancer status. The Kruskal–Wallis test was performed to compare TSS with a predominant pulmonary pattern. ROC curves were used to determine the best threshold or “cut-off” value for distinguishing between two categories. The Kaplan–Meier method was used to estimate the probability of survival. The survival distributions of two or more groups of a between-subjects factor were compared for equality using the log-rank test. Cox regression was used to investigate the effect of several variables on survival. For any of the used tests, results were considered statistically significant if p value ≤ 0.050.
Results
Patients’ characteristics
A total of 151 oncology patients with PCR-confirmed COVID-19 infection were included. The mortality rate was higher in patients older than 50 years. According to in-hospital mortality, 151 patients were classified into the non-survivor group (n = 77) and the survivor group (n = 74). The patients’ characteristics and risk factors for in-hospital mortality are illustrated in Table 1. Only 24 patients developed post-COVID-19 complications, including acute liver insult, acute renal failure, neurological complication (encephalopathy and coma), persistent thrombocytopenia and pulmonary complication (fungal infection and lung fibrosis). Regarding ECOG Performance status, pairwise comparisons showed a statistically significant difference between score 3 versus score 1 (χ2 = 10.561, p = 0.001), and score 2 versus score 1 (χ2 = 10.635, p = 0.001), but not between score 2 versus score 3. There was a statistically significant higher mortality in patients who required steroid use, mechanical ventilation and ICU admission. The median survival time (95% CI) was 60 (18–120) days (Additional file 1: Fig. S2).
Anticancer therapy
The most common malignancies were leukaemia 51 (33.7%) and breast cancer 31 (20.5%). The median duration between the last chemotherapy cycle and COVID-19 diagnosis was 10 days with the higher mortality rate in patients who received chemotherapy within 10 days before COVID-19 infection. There were no statistically significant differences in mortality regarding tumour type, anticancer therapy type, duration between last chemotherapy and COVID diagnosis if a patient was on active chemotherapy or not at the time of infection and post-COVID complication.
Cancer status and oncologic outcomes
Out of 85 patients who received chemotherapy before COVID-19 infection, 39 resumed chemotherapy after the resolution of COVID-19, while the other 46 patients died early. Other 18 newly diagnosed cancer patients started chemotherapy after the resolution of COVID-19. Post-COVID cancer status was accurately assessed in 130 patients, while 21 patients experienced rapid in-hospital mortality limiting their status assessment. Sixty-eight patients had tumour progression post-COVID-19 infection, 46 patients had tumour regression, and 16 patients were still in remission. There was a statistically significant higher mortality among patients with tumour stage 4 and patients with progressive disease (P < 0.001). Pairwise comparisons showed a statistically significant difference between progressive disease versus regressive disease (χ2 = 18.239, P < 0.001) and remittent disease (χ2 = 14.618, p < 0.001), but not between regressive versus remittent disease.
Chest CT findings
There was statistically significantly higher mortality among patients with consolidation, crazy paving pattern, pleural effusion and tree-in-bud pattern (Additional file 1: Fig. S3). All chest CT findings are illustrated in Table 2. The predominant pattern of abnormality upon hospital admission was pulmonary consolidation (71 patients) followed by GGO (68). Pairwise comparisons showed a statistically significant difference in mortality between GGO versus both consolidation (χ2 = 74.062, p < 0.001), and crazy paving (χ2 = 50.937, p < 0.001), but not between consolidation and crazy paving.
TSS
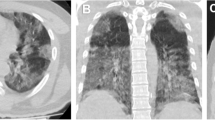
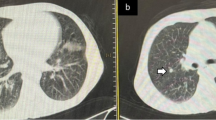
The ROC curve analysis revealed that TSS was statistically significantly higher in non-survivors (AUC = 0.758, p < 0.001). The optimum cut-off value of TSS used to predict in-hospital mortality was 5, with a sensitivity of 79.2% and a specificity of 62.2% (Fig. 1). Demonstrative survivor and non-survivor cases were presented in (Figs. 2, 3).
Median TSS (Q1–Q3) = 11.5 (7.3–14), 7 (5–10) and 5 (4–8) in patients with crazy paving, consolidation and GGO, respectively, with a statistically significant difference, H [2] = 22.556, P < 0.001. TSS was statistically significantly lower in GGO versus consolidation and crazy paving pattern (adjusted p = 0.001 and p < 0.001, respectively), but there was no statistically significant difference between TSS in consolidation and crazy paving pattern (adjusted p = 0.0156) (Additional file 1: Fig. S4).
There was no statistically significant difference in TSS among complicated and non-complicated cases (p = 0.473).
TSS correlation with treatment plan and cancer status
Median TSS (Q1–Q3) = 5 (4–7), 7.5 (5–10.3) and 5 (3–9.3) in patients who resumed, not resumed and started chemotherapy after COVID-19 treatment, respectively, with a statistically significant difference, H [2] = 11.53, P = 0.003). TSS was statistically significantly lower in patients who resumed versus who did not resume chemotherapy (adjusted p = 0.005), but there was no statistically significant difference between TSS in patients who resumed versus who started chemotherapy (adjusted p = 1). TSS was marginally significantly lower in patients who started versus those who did not resume chemotherapy (adjusted p = 0.057) (Additional file 1: Fig. S4).
There was a statistically significant positive correlation of large strength between the delay time to resume chemotherapy after COVID-19 resolution and TSS (n = 39), as TSS increased the time to resume chemotherapy is longer (rs = 0.511, p = 0.001). Also, there was a statistically significant positive correlation of low strength between the post-COVID-19 cancer status and TSS, as patients with progressive disease revealed higher TSS (rs = 0.183, p = 0.037).
Inter-observer reliability
Three hundred-two observations were depicted for the TSS. There was statistically significant reliability (absolute agreement) between the two independent observers in quantitative pulmonary assessment using TSS with ICC > 0.9 (P < 0.001) (Table 3 and Fig. 4).
Risk factors for mortality
The Cox regression analysis was performed to predict the risk factors for mortality. Univariate analysis revealed that age, pulmonary predominant pattern, pleural effusion, tree-in-bud, ECOG PS, tumour stage 4 and post-COVID cancer status were a statistically significant predictor of mortality. So, all these seven predictor variables were entered in a multivariate logistic regression model. The model was statistically significant (χ2 [7] = 75.79, p value < 0.001) with an AUC (95% CI) of 0.923 (0.863–0.963). Of the seven predictor variables, only consolidation versus GGO, crazy paving pattern versus GGO and progressive diseases versus remittent diseases were statistically significant independent predictors of mortality among oncology patients with COVID-19 infection. The tree-in-bud pattern was a marginally independent risk factor for mortality (Table 4).
Discussion
To the best of our knowledge, this is the first study to investigate the diagnostic reliability of TSS to predict mortality and its effect on treatment plans in oncology patients with COVID-19 infection. Our results revealed a statistically significant inter-observer agreement for the evaluation of pulmonary abnormalities with a statistically significantly higher TSS among non-survivors versus survivors. That was in line with a retrospective study limited to patients with solid malignancy concluded that patients who presented with high baseline CT scores and required ICU care had a higher mortality [23]. Unlike our study, there was no correlation between TSS and the implication of COVID-19 infection on the cancer status and management plan.
In concordance with our results, a recently published study discussed the severity of COVID-19 in oncology patients using chest radiographs only without performing chest CT and concluded that the baseline radiological severity of COVID-19 diagnosis is associated with poor outcomes [24].
Multivariate regression analysis revealed that post-COVID progressive cancer diseases versus remittent diseases were statistically significant independent predictors of mortality. Similar higher mortality in progressive disease was approved in a retrospective study on patients with solid malignancy, unlike our study there was no correlation between cancer status and TSS or even chest CT findings [23]. Our results revealed a statistically significant positive correlation between the post-COVID-19 cancer status and TSS. This is in concordance with the hypothesis that COVID-19 infection could be associated with proinflammatory markers which may activate premetastatic cancer cell dissemination [25, 26].
There were no statistically significant differences in mortality rate and TSS between complicated and non-complicated cases, thus assuming to be the small number of included complicated cases. Among the 24 complicated cases, 11 cases were died.
The management of patients in our centre was following multidisciplinary meeting decisions according to ESMO guidelines and treatment priorities in managing cancer patients during covid-19 pandemic [27, 28]. Regarding COVID-19 treatment, 89 patients received steroids with statistically higher mortality rates (p = 0.0025). A total of 111 patients did not receive antiviral drugs, while 10, 6, 3, 8, 7 and 5 patients received Acyclovir, Oseltamivir, Ribavirin, Favipiravir, Remdesivir and Avipravir, respectively.
There were no statistically significant differences regarding tumour type, anticancer therapy type and whether the patient was on active chemotherapy or not at the time of COVID-19 infection between survivors and non-survivors. This was in line with a previous study that discussed the determinants of COVID-19 severity in oncology patients [3]. Contrary to our results, studies in New York City cancer centre patients stated that patients with haematological tumours had a higher mortality rate than those with solid ones [29].
Our results revealed higher mortality in patients who received chemotherapy within 10 days before COVID-19 infection. In line with other studies concluded that anticancer therapy within 30 or 14 days before COVID-19 diagnosis has a higher risk of developing severe events [6].
Multivariate regression analysis revealed that consolidation or crazy paving pattern versus GGO were statistically significant independent predictors of mortality. There was no statistically significant difference in patients with GGO among survivors and non-survivors, while patients with consolidation or crazy paving patterns had a statistically significant higher mortality. This prevalence was in line with several previous studies on non-cancer patients [19, 30]. In our study predominant pulmonary pattern upon hospital admission was pulmonary consolidation followed by GGO, this high prevalence of consolidation could be explained by their immunocompromised status and rapid deterioration compared to the general population.
The GGO is considered a marker of the early and active disease stage [30]. Consolidation indicates an increased inflammatory exudate of the alveolar space with subsequent increased mortality, while the crazy paving pattern affirms a mixture of alveolar oedema and interstitial inflammatory changes [31].
Our results revealed a statistically significantly higher TSS among non-survivors versus survivors using a cut-off value of 5 or above for detection of in-hospital mortality. Concordant results were reported by a retrospective study in non-cancer patients with a higher TSS threshold of 7.5, 12 and 8.5 showed a sensitivity of 82.6%, 77.3% and 86.7% with a specificity of 100%, 90.5% and 87.5%, respectively, for diagnosing severe-critical COVID-19 cases and in-hospital mortality [19, 20, 22]. This lower TSS cut-off value in our patients is assumed to be the immune-compromised state and the detected high prevalence of predominant consolidation pattern, rationalizing more attention to those vulnerable patients. TSS was statistically significantly higher in patients with consolidation and crazy paving patterns versus patients with GGO.
So, we recommend that hospitalization and precise follow-up may be beneficial for oncology patients with a baseline TSS of more than 5. This can help rapid assessment and selection of proper treatment plans in critical cases, especially where ICU resources are restricted.
The chemotherapy was delayed in 39 patients, and TSS was statistically significantly lower in patients who resumed versus those who did not resume chemotherapy after COVID-19 resolution. Concordant results were revealed by a previous study on acute myeloid leukaemia patients as the chemotherapeutic schedule was delayed in 68 patients, unlike our study there was no correlation with TSS and treatment plan [32].
The strength of our study is besides the reasonable diagnostic performance of TSS to expect mortality, our results firstly presented a statistically significant positive correlation between TSS and post-COVID cancer status and delay in chemotherapy schedule. Thus, TSS provides an applicable rapid quantitative radiological assessment to predict mortality, cancer status and modification of treatment plan, helping to provide special cases in such settings and improve the patient’s outcome.
There are some limitations to the study. First, the single-centre retrospective study design is observed. Secondly, the number of complicated cases was small, further studies on post-COVID-19 complications are recommended. Lastly, future studies assessing the performance of machine-learning-based tools against radiologist-based scoring systems are recommended.
Conclusions
Predominant pulmonary patterns of consolidation or crazy paving pattern versus GGO and progressive diseases versus remittent diseases are statistically significant independent predictors of mortality in oncology patients. TSS assessed the severity of COVID-19 with excellent inter-observer agreement and revealed a positive correlation between delay time to resume chemotherapy and post-COVID cancer status. TSS is a rapidly applicable method to improve the decision-making about the treatment plan.
Availability of data and materials
All data are available upon request.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- CT:
-
Computed tomography
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- GGO:
-
Ground-glass opacity.
- ICC:
-
Interclass correlation
- RT-PCR:
-
Reverse transcription–polymerase chain reaction assay.
- TSS:
-
Total severity score
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J et al (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8):727–733
Al-Quteimat OM, Amer AM (2020) The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol 43:452–455
Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M et al (2020) Determinants of COVID-19 disease severity in patients with cancer. Nat Med 26(8):1218–1223
Group LCS. Chinese Thoracic Society, Chinese Medical Association; Chinese Respiratory Oncology Collaboration (2020) Expert recommendations on the management of patients with advanced non-small cell lung cancer during epidemic of COVID-19 (Trial version). Zhonghua Jie He He Hu Xi Za Zhi 43(4):297–301
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223):507–513
Liang W, Guan W, Chen R, Wang W, Li J, Xu K et al (2020) Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21(3):335–337
Cortellini A, Salazar R, Gennari A, Aguilar-Company J, Bower M, Bertuzzi A et al (2022) Persistence of long-term COVID-19 sequelae in patients with cancer: an analysis from the OnCovid registry. Eur J Cancer 170:10–16
Wong HYF, Lam HYS, Fong AH-T, Leung ST, Chin TW-Y, Lo CSY et al (2020) Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology 296(2):E72–E78
Wasilewski P, Mruk B, Mazur S, Półtorak-Szymczak G, Sklinda K, Walecki J (2020) COVID-19 severity scoring systems in radiological imaging—a review. Pol J Radiol 85(1):361–368
Hjouj M, Smerat S, Samra MA, Sada MG, Alarab A, Al Hroush IS et al (2021) Correlation of chest CT and RT-PCR testing for coronavirus disease 2020 (COVID-19) in Palestine: a report of 200 cases. Eur J Med Health Sci 3(2):38–41
Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W et al (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 296:E32–E40
Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N et al (2020) Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology 295:685–691
Kanne JP, Little BP, Chung JH, Elicker BM, Ketai LH (2020) Essentials for radiologists on COVID-19: an update—radiology scientific expert panel. Radiology 296:E113–E114
Raptis CA, Hammer MM, Short RG, Shah A, Bhalla S, Bierhals AJ et al (2020) Chest CT and coronavirus disease (COVID-19): a critical review of the literature to date. AJR Am J Roentgenol 215(4):839–842
Elmokadem AH, Bayoumi D, Abo-Hedibah SA, El-Morsy A (2021) Diagnostic performance of chest CT in differentiating COVID-19 from other causes of ground-glass opacities. Egypt J Radiol Nucl Med 52(1):1–10
Perrone F, Balbi M, Casartelli C, Buti S, Milanese G, Sverzellati N et al (2021) Differential diagnosis of COVID-19 at the chest computed tomography scan: a review with special focus on cancer patients. World J Radiol 13(8):243
Yang R, Li X, Liu H, Zhen Y, Zhang X, Xiong Q et al (2020) Chest CT severity score: an imaging tool for assessing severe COVID-19. Radiol Cardiothorac Imaging 2(2):e200047
Salaffi F, Carotti M, Tardella M, Borgheresi A, Agostini A, Minorati D et al (2020) The role of a chest computed tomography severity score in coronavirus disease 2019 pneumonia. Medicine 99(42):e22433
Elmokadem AH, Mounir AM, Ramadan ZA, Elsedeiq M, Saleh GA (2022) Comparison of chest CT severity scoring systems for COVID-19. Eur Radiol 32(5):3501–3512
Tharwat S, Saleh GA, Saleh M, Mounir AM, Abdelzaher DG, Salah AM et al (2022) Chest CT total severity score on admission to predict in-hospital mortality in COVID-19 patients with acute and chronic renal impairment. Diagnostics 12(7):1529
Ye Z, Zhang Y, Wang Y, Huang Z, Song B (2020) Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol 30(8):4381–4389
Li K, Fang Y, Li W, Pan C, Qin P, Zhong Y et al (2020) CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur Radiol 30(8):4407–4416
Narayan S, Talwar V, Goel V, Chaudhary K, Sharma A, Redhu P et al (2022) Co-relation of SARS-CoV-2 related 30-d mortality with HRCT score and RT-PCR Ct value-based viral load in patients with solid malignancy. World J Clin Oncol 13(5):339
Perez-Johnston R, Araujo-Filho J, Mckenney AS, Gangai N, Plodkowski AJ, Liu CC et al (2022) COVID-19 in patients with cancer: can baseline radiologic severity and early evolution predict clinical outcomes? Eur Radiol 32(4):2661–2671
Saha A, Anirvan P (2020) Cancer progression in COVID-19: integrating the roles of renin angiotensin aldosterone system, angiopoietin-2, heat shock protein-27 and epithelial mesenchymal transition. Ecancermedicalscience 14
De Winter FH, Hotterbeekx A, Huizing MT, Konnova A, Fransen E, Jongers BS et al (2021) Blood cytokine analysis suggests that SARS-CoV-2 infection results in a sustained tumour promoting environment in cancer patients. Cancers 13(22):5718
Curigliano G, Banerjee S, Cervantes A, Garassino M, Garrido P, Girard N et al (2020) Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol 31(10):1320–1335
Buske C, Dreyling M, Alvarez-Larrán A, Apperley J, Arcaini L, Besson C et al (2022) Managing hematological cancer patients during the COVID-19 pandemic: an ESMO-EHA Interdisciplinary Expert Consensus. ESMO open 7(2):100403
Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A et al (2020) Case fatality rate of cancer patients with COVID-19 in a New York Hospital SystemCase fatality rate of cancer patients with COVID-19. Cancer Discov 10(7):935–941
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (2020) Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 215(1):87–93
Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M et al (2020) Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol 33(6):1007–1014
Marchesi F, Salmanton-García J, Emarah Z, Piukovics K, Nucci M, López-García A et al (2020) COVID-19 in adult acute myeloid leukemia patients: a long-term followup study from the European Hematology Association survey (EPICOVIDEHA). Haematologica 108:22
Acknowledgements
Not applicable.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. G. S, A. M and M. E were responsible for radiological analysis of CT reports and made the score. M. S, M. H, S. A and D. S were responsible for collection of data. G. S wrote the manuscript. R. A shared in in the writing of the manuscript and final revision.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Institutional Review Board approval was obtained. (Approval No: R.22.06.1738), All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Consent of publication
Written informed consent was waived by the Institutional Review Board.
Competing interests
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1
. Flowchart of the study. Fig. S2. Kaplan–Meier curve for survival time analysis. Fig. S3. Kaplan–Meier curves of risk factors for mortality (A–F). Fig. S4. Pairwise comparisons of TSS and predominant pulmonary pattern as well as chemotherapy after COVID-19 (A&B).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saleh, G.A., Mounir, A.M., Elhawary, M.A. et al. Diagnostic reliability of chest CT qualitative and quantitative assessment to predict survival and morbidity in oncology patients with COVID-19 infection. Egypt J Radiol Nucl Med 55, 98 (2024). https://doi.org/10.1186/s43055-024-01259-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01259-2