Abstract
Objectives
To assess methods to improve the accuracy of prognosis for clinical stage I solid lung adenocarcinoma using radiomics based on different volumes of interests (VOIs).
Methods
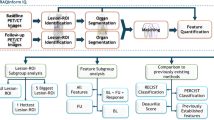
This retrospective study included patients with postoperative clinical stage I solid lung adenocarcinoma from two hospitals, center 1 and center 2. Three databases were generated: dataset A (training set from center 1), dataset B (internal test set from center 1), and dataset C (external validation test from center 2). Disease-free survival (DFS) data were collected. CT radiomics models were constructed based on four VOIs: gross tumor volume (GTV), 3 mm external to the tumor border (peritumoral volume [PTV]0~+3), 6 mm crossing tumor border (PTV−3~+3), and 6 mm external to the tumor border (PTV0~+6). The area under the receiver operating characteristic curve (AUC) was used to compare the model accuracies.
Results
A total of 334 patients were included (204 and 130 from centers 1 and 2). The model using PTV−3~+3 (AUC 0.81 [95% confidence interval {CI}: 0.75, 0.94], 0.81 [0.63, 0.90] for datasets B and C) outperformed the other three models, GTV (0.73 [0.58, 0.81], 0.73 [0.58, 0.83]), PTV0~+3 (0.76 [0.52, 0.87], 0.75 [0.60, 0.83]), and PTV0~+6 (0.72 [0.60, 0.81], 0.69 [0.59, 0.81]), in datasets B and C, all p < 0.05.
Conclusions
A radiomics model based on a VOI of 6 mm crossing tumor border more accurately predicts prognosis of clinical stage I solid lung adenocarcinoma than that based on VOIs including overall tumor or external rims of 3 mm and 6 mm.
Key Points
• Radiomics is a useful approach to improve the accuracy of prognosis for stage I solid adenocarcinoma.
• The radiomics model based on VOIs that includes 3 mm within and external to the tumor border (peritumoral volume [PTV] −3~+3 ) outperformed models that included either only the tumor itself or those that only included the peritumoral volume.






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the receiver operating characteristic curve
- CI :
-
Confidence interval
- DFS:
-
Disease-free survival
- GTV :
-
Gross tumor volume
- LASSO :
-
Least absolute shrinkage and selection operator
- PTV :
-
Peritumoral volume
- ROC :
-
Receiver operating characteristic curve
- ROI:
-
Region of interest
- VOIs:
-
Volumes of interests
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Chansky K, Detterbeck FC, Nicholson AG et al (2017) The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 12(7):1109–1121
al-Kattan K, Sepsas E, Fountain SW, Townsend ER (1997) Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg 12(3):380–384
Amin MB, Greene FL, Edge SB et al (2017) The eighth edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67:93–99
Burrell RA, Mc Granahan N, Bartek J, Swanton C (2013) The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501:338–345
Kadota K, J-i N, Sima CS, Sima RC et al (2015) Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 10:806–814
Shimada Y, Ishii G, Hishida T, Yoshida J, Nishimura M, Nagai K (2010) Extratumoral vascular invasion is a significant prognostic indicator and a predicting factor of distant metastasis in non-small cell lung cancer. J Thorac Oncol 5:970–975
Saijo T, Ishii G, Ochiai A et al (2007) Evaluation of extratumoral lymphatic permeation in non-small cell lung cancer as a means of predicting outcome. Lung Cancer 55:61–66
Kadota K, Yeh YC, Villena-Vargas J et al (2015) Tumor budding correlates with the protumor immune microenvironment and is an independent prognostic factor for recurrence of stage I lung adenocarcinoma. Chest 148:711–721
Chan R, He Y, Haque A, Zwischenberger J (2001) Computed tomographicpathologic correlation of gross tumor volume and clinical target volume in non-small cell lung cancer: a pilot experience. Arch Pathol Lab Med 125:1469–1472
Grills IS, Fitch DL, Goldstein NS et al (2007) Clinicopathologic analysis of microscopic extension in lung adenocarcinoma: defining clinical target volume for radiotherapy. Int J Radiat Oncol Biol Phys 69:334–341
Zhang WK, Xie LM, Wang ZS, Pang DQ (2017) Influence of microextension on clinical target volume delineation in adenocarcinoma and squamous cell carcinoma among patients with non-small cell lung cancer. Chin J Radiat Oncol 5:522–526
Bae JM, Jeong JY, Lee HY et al (2017) Pathologic stratification of operable lung adenocarcinoma using radiomics features extracted from dual energy CT images. Oncotarget 8:523–535
Huang Y, Liu Z, He L, Chen X et al (2016) Radiomics signature: a potential biomarker for the prediction of disease-free survival in early-stage (I or II) non-small cell lung cancer. Radiology 281:947–957
Beig N, Khorrami M, Alilou M et al (2018) Perinodular and intranodular radiomic features on lung CT images distinguish adenocarcinomas from granulomas. Radiology 290:783–792
Dou TH, Coroller TP, Van Griethuysen JJM et al (2018) Peritumoral radiomics features predict distant metastasis in locally advanced NSCLC. PLoS One 13(11):e0206108
Phillips I, Ajaz M, Ezhil V et al (2018) Clinical applications of textural analysis in non-small cell lung cancer. Br J Radiol 91(1081):20170267
Yip R, Ma T, Flores RM et al (2019) Survival with parenchymal and pleural invasion of non-small cell lung cancers less than 30 mm. J Thorac Oncol 14(5):890–902
Mao L, Chen H, Liang M et al (2019) Quantitative radiomic model for predicting malignancy of small solid pulmonary nodules detected by low-dose CT screening. Quant Imag Med Surg 9(2):263–272
Demler OV, Pencina MJ, D’agostino RB Sr (2012) Misuse of DeLong test to compare AUCs for nested models. Stat Med 31(23):2577–2587
Pietras K, Ostman A (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316(8):1324–1331
Tunali I, Hall LO, Napel S et al (2019) Stability and reproducibility of computed tomography radiomic features extracted from peritumoral regions of lung cancer lesions. Med Phys 46(11):5075–5085
Wang X, Zhao X, Li Q et al (2019) Can peritumoral radiomics increase the efficiency of the prediction for lymph node metastasis in clinical stage T1 lung adenocarcinoma on CT? Eur Radiol 29(11):6049–6058
Akinci D’antonoli T, Farchione A, Lenkowicz J et al (2020) CT radiomics signature of tumor and peritumoral lung parenchyma to predict nonsmall cell lung cancer postsurgical recurrence risk. Acad Radiol 27(4):497–507
Ruffini E, Asioli S, Filosso PL et al (2011) Significance of the presence of microscopic vascular invasion after complete resection of Stage I-II pT1-T2N0 non-small cell lung cancer and its relation with T-Size categories: did the 2009 7th edition of the TNM staging system miss something? J Thorac Oncol 6(2):319–326
Litjens G, Kooi T, Bejnordi BE et al (2017) A survey on deep learning in medical image analysis. Med Image Anal 42:60–88
Aokage K, Miyoshi T, Ishii G et al (2017) Clinical and pathological staging validation in the eighth edition of the TNM classification for lung cancer: correlation between solid size on thin-section computed tomography and invasive size in pathological findings in the new T classification. J Thorac Oncol 12(9):1403–1412
Huang YQ, Liang CH, He L et al (2016) Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol 34:2157–2164
Yu KH, Berry GJ, Rubin DL et al (2017) Association of omics features with histopathology patterns in lung adenocarcinoma. Cell Syst 5(6):620–627.e3
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577
Coroller TP, Agrawal V, Narayan V et al (2016) Radiomic phenotype features predict pathological response in non-small cell lung cancer. Radiother Oncol J 119:480–486
Traverso A, Wee L, Dekker A, Gillies R (2018) Repeatability and reproducibility of radiomic features: a systematic review. Int J Radiat Oncol Biol Phys 102(4):1143–1158
Grove O, Berglund AE, Schabath MB et al (2015) Quantitative computed tomographic descriptors associate tumor shape complexity and intratumor heterogeneity with prognosis in lung adenocarcinoma. PLoS One 10:e0118261
Hosny A, Parmar C, Coroller TP et al (2018) Deep learning for lung cancer prognostication: a retrospective multi-cohort radiomics study. PLoS Med 15:e1002711
Company names
Product IFoundry (Intelligence Foundry 1.1) GE Healthcare.
Funding
This study has received funding from the Guangdong Ministry of Education Industry-University-Research Project (2011A090200057).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Xueguo Liu.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in “Quantitative radiomic model for predicting malignancy of small solid pulmonary nodules detected by low-dose CT screening.”
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Kunfeng Liu and Kunwei Li are co-first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 360 kb)
Rights and permissions
About this article
Cite this article
Liu, K., Li, K., Wu, T. et al. Improving the accuracy of prognosis for clinical stage I solid lung adenocarcinoma by radiomics models covering tumor per se and peritumoral changes on CT. Eur Radiol 32, 1065–1077 (2022). https://doi.org/10.1007/s00330-021-08194-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08194-0