Abstract
Objectives
Accurate preoperative estimation of the risk of breast-conserving surgery (BCS) resection margin positivity would be beneficial to surgical planning. In this multicenter validation study, we developed an MRI-based radiomic model to predict the surgical margin status.
Methods
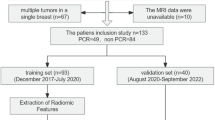
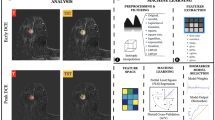
We retrospectively collected preoperative breast MRI of patients undergoing BCS from three hospitals (SYMH, n = 296; SYSUCC, n = 131; TSPH, n = 143). Radiomic-based model for risk prediction of the margin positivity was trained on the SYMH patients (7:3 ratio split for the training and testing cohorts), and externally validated in the SYSUCC and TSPH cohorts. The model was able to stratify patients into different subgroups with varied risk of margin positivity. Moreover, we used the immune-radiomic models and epithelial-mesenchymal transition (EMT) signature to infer the distribution patterns of immune cells and tumor cell EMT status under different marginal status.
Results
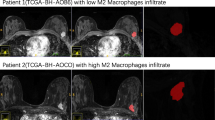
The AUCs of the radiomic-based model were 0.78 (0.66–0.90), 0.88 (0.79–0.96), and 0.76 (0.68–0.84) in the testing cohort and two external validation cohorts, respectively. The actual margin positivity rates ranged between 0–10% and 27.3–87.2% in low-risk and high-risk subgroups, respectively. Positive surgical margin was associated with higher levels of EMT and B cell infiltration in the tumor area, as well as the enrichment of B cells, immature dendritic cells, and neutrophil infiltration in the peritumoral area.
Conclusions
This MRI-based predictive model can be used as a reliable tool to predict the risk of margin positivity of BCS. Tumor immune-microenvironment alteration was associated with surgical margin status.
Clinical relevance statement
This study can assist the pre-operative planning of BCS. Further research on the tumor immune microenvironment of different resection margin states is expected to develop new margin evaluation indicators and decipher the internal mechanism.
Key Points
• The MRI-based radiomic prediction model (CSS model) incorporating features extracted from multiple sequences and segments could estimate the margin positivity risk of breast-conserving surgery.
• The radiomic score of the CSS model allows risk stratification of patients undergoing breast-conserving surgery, which could assist in surgical planning.
• With the help of MRI-based radiomics to estimate the components of the immune microenvironment, for the first time, it is found that the margin status of breast-conserving surgery is associated with the infiltration of immune cells in the microenvironment and the EMT status of breast tumor cells.






Similar content being viewed by others
Abbreviations
- BCS:
-
Breast-conserving surgery
- CRC:
-
Colorectal cancer
- CSS model:
-
Comb_Seg_Seq model
- DCIS:
-
Ductal carcinoma in situ
- DEGs:
-
Differentially expressed genes
- EMT:
-
Epithelial-mesenchymal transition
- ER:
-
Estrogen receptor
- EIC:
-
Extensive intraductal component
- FSA:
-
Frozen-section analysis
- HER-2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hormone receptor
- iDCs:
-
Immature dendritic cells
- NPV:
-
Negative predictive value
- PR:
-
Progesterone receptor
- RC model:
-
Radiomics-Clinicopathological Model
- RFE:
-
Recursive feature elimination
- TB:
-
Tumor budding
- TCGA:
-
The Cancer Genome Atlas
- TiME:
-
Tumor immune microenvironment
References
Fisher B, Anderson S, Bryant J et al (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233–1241
Veronesi U, Cascinelli N, Mariani L et al (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347:1227–1232
Houssami N, Macaskill P, Marinovich ML, Morrow M (2014) The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol 21:717–730
Morrow M, Harris JR, Schnitt SJ (2012) Surgical margins in lumpectomy for breast cancer–bigger is not better. N Engl J Med 367:79–82
Mihalcik SA, Rawal B, Braunstein LZ et al (2017) The impact of reexcision and residual disease on local recurrence following breast-conserving therapy. Ann Surg Oncol 24:1868–1873
McCahill LE, Single RM, Aiello Bowles EJ et al (2012) Variability in reexcision following breast conservation surgery. JAMA 307:467–475
Wilke LG, Czechura T, Wang C et al (2014) Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the National Cancer Data Base, 2004–2010. JAMA Surg 149:1296–1305
Landercasper J, Attai D, Atisha D et al (2015) Toolbox to reduce lumpectomy reoperations and improve cosmetic outcome in breast cancer patients: the American Society of Breast Surgeons Consensus Conference. Ann Surg Oncol 22:3174–3183
O’Kelly Priddy CM, Forte VA, Lang JE (2016) The importance of surgical margins in breast cancer. J Surg Oncol 113:256–263
Nowikiewicz T, Śrutek E, Głowacka-Mrotek I, Tarkowska M, Żyromska A, Zegarski W (2019) Clinical outcomes of an intraoperative surgical margin assessment using the fresh frozen section method in patients with invasive breast cancer undergoing breast-conserving surgery - a single center analysis. Sci Rep 9:13441
Harness JK, Giuliano AE, Pockaj BA, Downs-Kelly E (2014) Margins: a status report from the Annual Meeting of the American Society of Breast Surgeons. Ann Surg Oncol 21:3192–3197
Rosenberger LH, Mamtani A, Fuzesi S et al (2016) Early adoption of the SSO-ASTRO consensus guidelines on margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: initial experience from Memorial Sloan Kettering Cancer Center. Ann Surg Oncol 23:3239–3246
van Deurzen CH (2016) Predictors of surgical margin following breast-conserving surgery: a large population-based cohort study. Ann Surg Oncol 23:627–633
Alves-Ribeiro L, Osório F, Amendoeira I, Fougo JL (2016) Positive margins prediction in breast cancer conservative surgery: assessment of a preoperative web-based nomogram. Breast 28:167–173
Barentsz MW, Postma EL, van Dalen T et al (2015) Prediction of positive resection margins in patients with non-palpable breast cancer. Eur J Surg Oncol 41:106–112
Jung JJ, Kang E, Kim EK et al (2020) External validation and modification of nomogram for predicting positive resection margins before breast conserving surgery. Breast Cancer Res Treat 183:373–380
Zhao R, Xing J, Gao J (2022) Development and validation of a prediction model for positive margins in breast-conserving surgery. Front Oncol 12:875665
Balleyguier C, Dunant A, Ceugnart L et al (2019) Preoperative breast magnetic resonance imaging in women with local ductal carcinoma in situ to optimize surgical outcomes: results from the randomized phase III trial IRCIS. J Clin Oncol 37:885–892
Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446
Conti A, Duggento A, Indovina I, Guerrisi M, Toschi N (2021) Radiomics in breast cancer classification and prediction. Semin Cancer Biol 72:238–250
Liu Z, Li Z, Qu J et al (2019) Radiomics of multiparametric MRI for pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Clin Cancer Res 25:3538–3547
Liu Z, Feng B, Li C et al (2019) Preoperative prediction of lymphovascular invasion in invasive breast cancer with dynamic contrast-enhanced-MRI-based radiomics. J Magn Reson Imaging 50:847–857
Hectors SJ, Lewis S, Besa C et al (2020) MRI radiomics features predict immuno-oncological characteristics of hepatocellular carcinoma. Eur Radiol 30:3759–3769
Kim AR, Choi KS, Kim MS et al (2021) Absolute quantification of tumor-infiltrating immune cells in high-grade glioma identifies prognostic and radiomics values. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-020-02836-w
Sun R, Limkin EJ, Vakalopoulou M et al (2018) A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol 19:1180–1191
Chen K, Jia W, Li S et al (2011) Cavity margin status is an independent risk factor for local-regional recurrence in breast cancer patients treated with neoadjuvant chemotherapy before breast-conserving surgery. Am Surg 77:1700–1706
Chen K, Zeng Y, Jia H et al (2012) Clinical outcomes of breast-conserving surgery in patients using a modified method for cavity margin assessment. Ann Surg Oncol 19:3386–3394
Chen K, Liu JQ, Wu W et al (2021) Clinical practice guideline for breast-conserving surgery in patients with early-stage breast cancer: Chinese Society of Breast Surgery (CSBrS) practice guidelines 2021. Chin Med J (Engl) 134:2143–2146
Allison KH, Hammond MEH, Dowsett M et al (2020) Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol 38:1346–1366
Wolff AC, Hammond MEH, Allison KH et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline focused update. J Clin Oncol 36:2105–2122
Johnston SRD, Harbeck N, Hegg R et al (2020) Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 38:3987–3998
Harbeck N, Rastogi P, Martin M et al (2021) Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol 32:1571–1581
Tustison NJ, Avants BB, Cook PA et al (2010) N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29:1310–1320
Fedorov A, Beichel R, Kalpathy-Cramer J et al (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30:1323–1341
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107
Aran D, Hu Z, Butte AJ (2017) xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol 18:220
Cañadas I, Rojo F, Taus Á et al (2014) Targeting epithelial-to-mesenchymal transition with Met inhibitors reverts chemoresistance in small cell lung cancer. Clin Cancer Res 20:938–950
Klinke DJ 2nd, Torang A (2020) An unsupervised strategy for identifying epithelial-mesenchymal transition state metrics in breast cancer and melanoma. iScience 23:101080
Shin HC, Han W, Moon HG et al (2012) Nomogram for predicting positive resection margins after breast-conserving surgery. Breast Cancer Res Treat 134:1115–1123
Pleijhuis RG, Kwast AB, Jansen L et al (2013) A validated web-based nomogram for predicting positive surgical margins following breast-conserving surgery as a preoperative tool for clinical decision-making. Breast 22:773–779
Pan Z, Zhu L, Li Q et al (2018) Predicting initial margin status in breast cancer patients during breast-conserving surgery. Onco Targets Ther 11:2627–2635
Houssami N, Ciatto S, Ellis I, Ambrogetti D (2007) Underestimation of malignancy of breast core-needle biopsy: concepts and precise overall and category-specific estimates. Cancer 109:487–495
Knuttel FM, Menezes GL, van Diest PJ, Witkamp AJ, van den Bosch MA, Verkooijen HM (2016) Meta-analysis of the concordance of histological grade of breast cancer between core needle biopsy and surgical excision specimen. Br J Surg 103:644–655
Chong HH, Yang L, Sheng RF et al (2021) Multi-scale and multi-parametric radiomics of gadoxetate disodium-enhanced MRI predicts microvascular invasion and outcome in patients with solitary hepatocellular carcinoma ≤ 5 cm. Eur Radiol 31:4824–4838
Li R (2020) Peritumoral radiomics and predicting treatment response. JAMA Netw Open 3:e2016125
Li C, Song L, Yin J (2021) Intratumoral and peritumoral radiomics based on functional parametric maps from breast DCE-MRI for prediction of HER-2 and Ki-67 status. J Magn Reson Imaging 54:703–714
He D, Wang X, Fu C et al (2021) MRI-based radiomics models to assess prostate cancer, extracapsular extension and positive surgical margins. Cancer Imaging 21:46
Lee S, Jung JY, Nam Y et al (2022) Diagnosis of marginal infiltration in soft tissue sarcoma by radiomics approach using T2-weighted Dixon sequence. J Magn Reson Imaging. https://doi.org/10.1002/jmri.28331
Dongre A, Weinberg RA (2019) New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20:69–84
Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID (2021) Tumour budding in solid cancers. Nat Rev Clin Oncol 18:101–115
Lee H, Sha D, Foster NR et al (2020) Analysis of tumor microenvironmental features to refine prognosis by T, N risk group in patients with stage III colon cancer (NCCTG N0147) (Alliance). Ann Oncol 31:487–494
Lloyd AJ, Ryan ÉJ, Boland MR et al (2020) The histopathological and molecular features of breast carcinoma with tumour budding-a systematic review and meta-analysis. Breast Cancer Res Treat 183:503–514
Petrova E, Zielinski V, Bolm L et al (2020) Tumor budding as a prognostic factor in pancreatic ductal adenocarcinoma. Virchows Arch 476:561–568
Ueno H, Ishiguro M, Nakatani E et al (2019) Prospective multicenter study on the prognostic and predictive impact of tumor budding in stage II colon cancer: results from the SACURA trial. J Clin Oncol 37:1886–1894
Lugli A, Kirsch R, Ajioka Y et al (2017) Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 30:1299–1311
Bell D, Chomarat P, Broyles D et al (1999) In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med 190:1417–1426
Fainaru O, Almog N, Yung CW et al (2010) Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. FASEB J 24:1411–1418
Meng F, Li W, Li C, Gao Z, Guo K, Song S (2015) CCL18 promotes epithelial-mesenchymal transition, invasion and migration of pancreatic cancer cells in pancreatic ductal adenocarcinoma. Int J Oncol 46:1109–1120
Li S, Cong X, Gao H et al (2019) Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res 38:6
Kang W, Feng Z, Luo J et al (2021) Tertiary lymphoid structures in cancer: the double-edged sword role in antitumor immunity and potential therapeutic induction strategies. Front Immunol 12:689270
Moran MS, Schnitt SJ, Giuliano AE et al (2014) Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol 32:1507–1515
Chen JY, Huang YJ, Zhang LL, Yang CQ, Wang K (2018) Comparison of oncoplastic breast-conserving surgery and breast-conserving surgery alone: a meta-analysis. J Breast Cancer 21:321–329
Li M, Xu B, Shao Y, Liu H, Du B, Yuan J (2017) Magnetic resonance imaging patterns of tumor regression in breast cancer patients after neo-adjuvant chemotherapy, and an analysis of the influencing factors. Breast J 23:656–662
Acknowledgements
We appreciate the assistance from the Disease Registry Department, the Artificial Intelligence Lab and the Big Data Center of Sun Yat-sen Memorial Hospital, Sun Yat-sen University. We also appreciate the support from REDCap development team and research teams of Vanderbilt University Medical Center.
Funding
This work was supported by grants from the Natural Science Foundation of China (81621004, 92159303, 81720108029, 81930081, 91940305), Guangdong Science and Technology Department (2020B1212060018, 2020B1212030004, 2019A1515110075), Department of Natural Resources of Guangdong Province (GDNRC[2021]51), Clinical Innovation Research Program of Bioland Laboratory (2018GZR0201004), Bureau of Science and Technology of Guangzhou (20212200003, 202102010221), the Program for Guangdong Introducing Innovative and Enterpreneurial Teams (2019BT02Y198), the Yat-sen Scholarship of Young Scientist program of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, and by the grants from the Sun Yat-sen Clinical Research Cultivating Program of Sun Yat-sen Memorial Hospital, Sun Yat-sen University(#SYS-Q-202002), the Sun Yat-Sen University Clinical Research 5010 Program (#2018022), as well as by National Natural Science Foundation of Guangdong Province (#2019A1515011467, #2021A1515012361, #2019A1515110075), grants from the National Institute of Hospital Administration (#RXDBZ-2022-04) and the grants from the China Anti-aging Promoting Association.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Erwei Song.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
The requirement for informed consent was waived and patient data are anonymized.
Ethical approval
The study was approved by the IRBs of the three included hospitals, respectively. Among them, the approval number of Sun Yat-sen Memorial Hospital of Sun Yat-sen University (SYMH) IRB is 2020-KY-028, the approval number of Sun Yat-sen University Cancer Center (SYSUCC) IRB is B2020-333-01, and the approval number of Tangshan People’s Hospital (TSPH) IRB is RMYY-LLKS-2020-104.
Study subjects or cohorts overlap
Study subjects or cohorts have not been previously reported.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, J., Chen, K., Li, S. et al. MRI-based radiomic models to predict surgical margin status and infer tumor immune microenvironment in breast cancer patients with breast-conserving surgery: a multicenter validation study. Eur Radiol 34, 1774–1789 (2024). https://doi.org/10.1007/s00330-023-10144-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10144-x