Abstract
Objectives
To assess the efficacy of diffusion kurtosis imaging (DKI) in differentiating low-grade from high-grade tumors and evaluating the aggressiveness of bladder cancer.
Methods
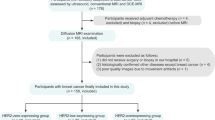
From January 2017 to July 2017, 35 patients (28 males, 7 females; mean age 63 ± 9 years) diagnosed with bladder cancer underwent diffusion-weighted imaging (DWI) with two types of DKI protocols: (1) multi-b value ranging from 0 to 2000 s/mm2 to obtain mean diffusivity/kurtosis (MDb/MKb) and (2) the tensor method with 32 directions with 3 b values (0, 1000, and 2000s/mm2) to obtain mean/axial/radial diffusivity (MDt/Da/Dr), mean/axial/radial kurtosis (MKt/Ka/Kr), and fractional anisotropy (FA) before radical cystectomy. Comparisons between the low- and high-grade groups, non-muscle-invasive bladder cancer (NMIBC), and muscle-invasive bladder cancer (MIBC) were performed with the areas under the receiver operating characteristic curves (AUCs).
Results
The MKt and Kr values were significantly (p = 0.017 and p = 0.048) higher in patients with high-grade bladder tumors than in those with low-grade tumors. The MKt, Kr, and MKb values were significantly (p = 0.022, p = 0.000, and p = 0.044, respectively) higher in patients with MIBC than in those with NMIBC, while no significant differences (p > 0.05) were found in other values. The AUC of Kr (0.883) was the largest and was significantly higher than those of other metrics (all p < 0.05) for differentiating MIBC from NMIBC, with a sensitivity and specificity of 81.8% and 91.7%, respectively.
Conclusions
Kurtosis metrics performed better than diffusion metrics in differentiating MIBC from NMIBC, and directional kurtosis and Kr metrics may also have great potential in providing additional information regarding bladder cancer invasiveness.
Key Points
• Kurtosis metrics performed better than diffusion metrics in differentiating muscle-invasive bladder cancer (MIBC) from non-muscle-invasive bladder cancer (NMIBC).
• Directional kurtosis can provide additional directional microstructural information regarding bladder cancer invasiveness.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the receiver operating characteristic curve
- CLLS:
-
Constrained linear least-square
- Da:
-
Axial diffusivity
- DKT:
-
Diffusion kurtosis tensor
- Dr.:
-
Radial diffusivity
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion-weighted imaging
- FA:
-
Fractional anisotropy
- ICC:
-
Intraclass correlation coefficient
- Ka:
-
Axial kurtosis
- Kr:
-
Radial kurtosis
- MIBC:
-
Muscle-invasive bladder cancer
- MK:
-
Mean kurtosis
- MRI:
-
Magnetic resonance imaging
- NMIBC:
-
Non-muscle-invasive bladder cancer
- ROC:
-
Receiver operator characteristic
- TUR:
-
Transurethral resection
References
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Rabie E, Faeghi F, Izadpanahi MH, Dayani MA (2016) Role of dynamic contrast-enhanced magnetic resonance imaging in staging of bladder cancer. J Clin Diagn Res 10:TC01–TC05
Takeuchi M, Sasaki S, Ito M et al (2009) Urinary bladder cancer: diffusion-weighted MR imaging--accuracy for diagnosing T stage and estimating histologic grade. Radiology 251:112–121
Rosenkrantz AB, Mussi TC, Spieler B, Melamed J, Taneja SS, Huang WC (2012) High-grade bladder cancer: association of the apparent diffusion coefficient with metastatic disease: preliminary results. J Magn Reson Imaging 35:1478–1483
Dağgülli M, Onur MR, Fırdolaş F, Onur R, Kocakoç E, Orhan İ (2011) Role of diffusion MRI and apparent diffusion coefficient measurement in the diagnosis, staging and pathological classification of bladder tumors. Urol Int 87:346–352
Takeuchi M, Sasaki S, Naiki T et al (2013) MR imaging of urinary bladder cancer for T-staging: a review and a pictorial essay of diffusion-weighted imaging. J Magn Reson Imaging 38:1299–1309
Kobayashi S, Koga F, Kajino K et al (2014) Apparent diffusion coefficient value reflects invasive and proliferative potential of bladder cancer. J Magn Reson Imaging 39:172–178
El-Assmy A, Abou-El-Ghar ME, Refaie HF, Mosbah A, El-Diasty T (2012) Diffusion-weighted magnetic resonance imaging in follow-up of superficial urinary bladder carcinoma after transurethral resection: initial experience. BJU Int 110:E622–E627
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53:1432–1440
Tabesh A, Jensen JH, Ardekani BA, Helpern JA (2011) Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med 65:823–836
Jensen JH, Helpern JA (2010) MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 23:698–710
O'Brien T, Cranston D, Fuggle S, Bicknell R, Harris AL (1995) Different angiogenic pathways characterize superficial and invasive bladder cancer. Cancer Res 55:510–513
Van Cauter S, Veraart J, Sijbers J et al (2012) Gliomas: diffusion kurtosis MR imaging in grading. Radiology 263:492–501
Tietze A, Hansen MB, Østergaard L et al (2015) Mean diffusional kurtosis in patients with glioma: initial results with a fast imaging method in a clinical setting. AJNR Am J Neuroradiol 36:1472–1478
May M, Brookman-Amissah S, Roigas J et al (2010) Prognostic accuracy of individual uropathologists in noninvasive urinary bladder carcinoma: a multicentre study comparing the 1973 and 2004 World Health Organisation classifications. Eur Urol 57:850–858
Nogueira L, Brandão S, Matos E et al (2014) Application of the diffusion kurtosis model for the study of breast lesions. Eur Radiol 24:1197–1203
Suo S, Chen X, Wu L et al (2014) Non-Gaussian water diffusion kurtosis imaging of prostate cancer. Magn Reson Imaging 32:421–427
Rosenkrantz AB, Sigmund EE, Johnson G et al (2012) Prostate cancer: feasibility and preliminary experience of a diffusional kurtosis model for detection and assessment of aggressiveness of peripheral zone cancer. Radiology 264:126–135
Roethke MC, Kuder TA, Kuru TH et al (2015) Evaluation of diffusion kurtosis imaging versus standard diffusion imaging for detection and grading of peripheral zone prostate cancer. Invest Radiol 50:483–489
Suo S, Chen X, Ji X et al (2015) Investigation of the non-Gaussian water diffusion properties in bladder cancer using diffusion kurtosis imaging: a preliminary study. J Comput Assist Tomogr 39:281–285
Jensen JH, Falangola MF, Hu C et al (2011) Preliminary observations of increased diffusional kurtosis in human brain following recent cerebral infarction. NMR Biomed 24:452–457
Pentang G, Lanzman RS, Heusch P et al (2014) Diffusion kurtosis imaging of the human kidney: a feasibility study. Magn Reson Imaging 32:413–420
Veraart J, Poot DH, Van Hecke W et al (2011) More accurate estimation of diffusion tensor parameters using diffusion kurtosis imaging. Magn Reson Med 65:138–145
Quentin M, Pentang G, Schimmöller L et al (2014) Feasibility of diffusional kurtosis tensor imaging in prostate MRI for the assessment of prostate cancer: preliminary results. Magn Reson Imaging 32:880–885
Van Camp N, Blockx I, Verhoye M et al (2009) Diffusion tensor imaging in a rat model of Parkinson’s disease after lesioning of the nigrostriatal tract. NMR Biomed 22:697–706
Hui ES, Cheung MM, Qi L, Wu EX (2008) Towards better MR characterization of neural tissues using directional diffusion kurtosis analysis. Neuroimage 42:122–134
Wu G, Zhao Z, Yao Q et al (2018) The study of clear cell renal cell carcinoma with MR diffusion kurtosis tensor imaging and its histopathologic correlation. Acad Radiol 25:430–438
Priola AM, Priola SM, Gned D et al (2017) Diffusion-weighted quantitative MRI of pleural abnormalities: intra- and interobserver variability in the apparent diffusion coefficient measurements. J Magn Reson Imaging 46:769–782
Funding
This study has received funding from the National Natural Science Foundation of China (Youth Program Nos. 81601487, 81672514).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jian-Rong Xu.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Study subjects or cohorts overlap have not been published previously and not under consideration for publication elsewhere, in whole or in part.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, F., Chen, HG., Zhang, RY. et al. Diffusion kurtosis imaging to assess correlations with clinicopathologic factors for bladder cancer: a comparison between the multi-b value method and the tensor method. Eur Radiol 29, 4447–4455 (2019). https://doi.org/10.1007/s00330-018-5977-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5977-y