Abstract
Objectives
To date, there is no approved second-line treatment for patients dismissing sorafenib or ineligible for this treatment, so it would be useful to find an effective alternative treatment option. The aim of our study was to evaluate safety, feasibility and effectiveness of transarterial chemoembolisation with degradable starch microspheres (DSM-TACE) in the treatment of patients with advanced hepatocellular carcinoma (HCC) dismissing or ineligible for multikinase-inhibitor chemotherapy administration (sorafenib) due to unbearable side effects or clinical contraindications.
Methods
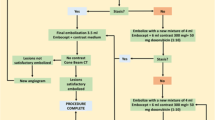
Forty consecutive BCLC stage B or C patients (31 male; age, 70.6 ± 13.6 years), with intermediate or locally advanced HCC dismissing or ineligible for sorafenib administration, who underwent DSM-TACE treatment cycle via lobar approach were prospectively enrolled. Tumour response was evaluated on multidetector computed tomography based on mRECIST criteria. Primary endpoints were safety, tolerance and overall disease control (ODC); secondary endpoints were progression-free survival (PFS) and overall survival (OS).
Results
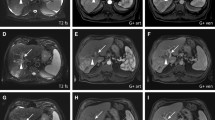
Technical success was achieved in all patients. No intra/peri-procedural death/major complications occurred. No signs of liver failure or systemic toxicity were detected. At 1-year follow-up, ODC of 52.5% was registered. PFS was 6.4 months with a median OS of 11.3 months.
Conclusions
DSM-TACE is safe and effective as a second-line treatment in HCC patients dismissing or ineligible for sorafenib.
Key Points
• DSM-TACE is safe and effective as second-line treatment in HCC patients dismissing or ineligible for sorafenib
• DSM-TACE allows the temporary occlusion of the smaller arterial vessels, improving overall therapeutic effectiveness by reducing the immediate wash-out of the cytostatic agent
• DSM-TACE also decreases the risk of systemic toxicity and post-embolic syndrome

Similar content being viewed by others
Abbreviations
- BCLC-B:
-
Barcelona Clinic Liver Cancer stage B
- BCLC-C:
-
Barcelona Clinic Liver Cancer stage C
- CR:
-
Complete response
- DSM-TACE:
-
Degradable starch microspheres – transarterial chemoembolisation
- ECOG:
-
Eastern Cooperative Oncology Group
- HCC:
-
Hepatocellular carcinoma
- JSH:
-
Japan Society of Hepatology
- ODC:
-
Overall disease control
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- PR:
-
Partial response
- SD:
-
Stable disease
- SIRT:
-
Selective internal radiotherapy
References
Rinninella E, Zocco MA, De Gaetano A et al (2012) From small nodule to overt HCC: a multistep process of carcinogenesis as seen during surveillance. Eur Rev Med Pharmacol Sci 16:1292–1294
Murata S, Mine T, Sugihara F et al (2014) Interventional treatment for unresectable hepatocellular carcinoma. World J Gastroenterol 20:13453–13465
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Llovet JM, Brú C, Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19:329–338
Falkson G, MacIntyre JM, Moertel CG, Johnson LA, Scherman RC (1984) Primary liver cancer. An Eastern Cooperative Oncology Group Trial. Cancer 54:970–977
Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C (2010) A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 51:1274–1283
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Cheng AL, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Italian Association for the Study of the Liver (AISF); AISF Expert Panel; AISF Coordinating Committee, Bolondi L, Cillo U, Colombo M et al (2013) Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis 45:712–723
Iavarone M, Cabibbo G, Piscaglia F et al (2011) Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology 54:2055–2063
Ponziani FR, Bhoori S, Germini A et al (2016) Inducing tolerability of adverse events increases sorafenib exposure and optimizes patient's outcome in advanced hepatocellular carcinoma. Liver Int 36:1033–1042
Niessen C, Unterpaintner E, Goessmann H et al (2014) Degradable starch microspheres versus ethidol and doxorubicin in transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol 25:240–247
Yamasaki T, Hamabe S, Saeki I et al (2011) A novel transcatheter arterial infusion chemotherapy using iodized oil and degradable starch microspheres for hepatocellular carcinoma: a prospective randomized trial. J Gastroenterol 46:359–366
Iezzi R, Pompili M, Nestola M et al (2016) Transarterial chemoembolization with degradable starch microspheres (DSM-TACE): an alternative option for advanced HCC patients? Preliminary results. Eur Rev Med Pharmacol Sci 20:2872–2877
Schicho A, Pereira PL, Haimerl M et al (2017) Transarterial chemoembolization (TACE) with degradable starch microspheres (DSM) in hepatocellular carcinoma (HCC): multi-center results on safety and efficacy. Oncotarget 8:72613–72620
Kudo M, Izumi N, Kokudo N et al (2011) HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 29:339–364
Kudo M, Matsui O, Izumi N et al (2014) Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 87:22–31
Ikai I, Kudo M, Arii S et al (2010) Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res 40:1043–1059
Costentin CE, Ferrone CR, Arellano RS, Ganguli S, Hong TS, Zhu AX (2017) Hepatocellular carcinoma with macrovascular invasion: defining the optimal treatment strategy. Liver Cancer 6:360–374
Basile A, Carrafiello G, Ierardi AM, Tsetis D, Brountzos E (2012) Quality-improvement guidelines for hepatic transarterial chemoembolization. Cardiovasc Intervent Radiol 35:765–774
Bruix J, Sherman M, Practice Guidelines Committee, American Association for the Study of Liver Diseases (2005) Management of hepatocellular carcinoma. Hepatology 42:1208–1236
Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK (2005) Systematic review: The model for end-stage liver disease–should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther 22:1079–1089
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Terashima T, Yamashita T, Arai K et al (2014) Feasibility and efficacy of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma after sorafenib. Hepatol Res 44:1179–1185
Finn RS, Kang YK, Mulcahy M et al (2012) Phase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res 18:2090–2098
Santoro A, Rimassa L, Borbath I et al (2013) Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomized, placebo-controlled phase 2 study. Lancet Oncol 14:55–63
Yau T, Wong H, Chan P et al (2012) Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Invest New Drugs 30:2384–2390
Bruix J, Qin S, Merle P et al (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:56–66
El-Khoueiry AB, Sangro B, Yau T et al (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389:2492–2502
Wiggermann P, Wohlgemuth WA, Heibl M et al (2013) Dynamic evaluation and quantification of microvascularization during degradable starch microspheres transarterial Chemoembolisation (DSM-TACE) of HCC lesions using contrast enhanced ultrasound (CEUS): a feasibility study. Clin Hemorheol Microcirc 53:337–348
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer (2012) EASL–EORTC Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Giannini EG, Bucci L, Garuti F et al (2018) Patients with advanced hepatocellular carcinoma need a personalized management: A lesson from clinical practice. Hepatology 67(5):1784–1796
Bruix J, Llovet JM (2002) Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 35:519–524
Greten TF, Papendorf F, Bleck JS et al (2005) Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer 92:1862–1868
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Roberto Iezzi, MD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• prospective
• experimental
• performed at one institution
Rights and permissions
About this article
Cite this article
Iezzi, R., Pompili, M., Rinninella, E. et al. TACE with degradable starch microspheres (DSM-TACE) as second-line treatment in HCC patients dismissing or ineligible for sorafenib. Eur Radiol 29, 1285–1292 (2019). https://doi.org/10.1007/s00330-018-5692-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5692-8