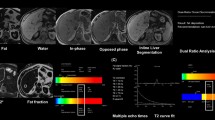
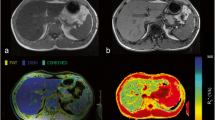
Abstract
Objectives
To determine the feasibility of a multi-step magnetic resonance imaging (MRI) approach for comprehensive assessment of hepatic steatosis defined as liver fat content of ≥5 % in an asymptomatic population.
Methods
The study was approved by the institutional review board and written informed consent of all participants was obtained. Participants of a population-based study cohort underwent a three-step 3-T MRI-based assessment of liver fat. A dual-echo Dixon sequence was performed to identify subjects with hepatic steatosis, followed by a multi-echo Dixon sequence with proton density fat fraction estimation. Finally, single-voxel T2-corrected multi-echo spectroscopy was performed.
Results
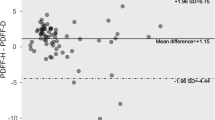
A total of 215 participants completed the MRI protocol (56.3 % male, average age 57.2 ± 9.4 years). The prevalence of hepatic steatosis was 55 %. Mean liver proton density fat fraction was 9.2 ± 8.5 % by multi-echo Dixon and 9.3 ± 8.6 % by multi-echo spectroscopy (p = 0.51). Dual-echo Dixon overestimated liver fat fraction by 1.4 ± 2.0 % (p < 0.0001). All measurements showed excellent correlations (r ≥ 0.9, p < 0.001). Dual-echo Dixon was highly sensitive for the detection of hepatic steatosis (sensitivity 0.97, NPV 0.96) with good specificity and PPV (0.75 and 0.81, respectively).
Conclusions
A multi-step MRI approach may enable rapid and accurate identification of subjects with hepatic steatosis in an asymptomatic population.
Key Points
• Dual-echo Dixon can rapidly and reliably exclude hepatic steatosis without complex post-processing.
• Multi-echo Dixon and multi-echo spectroscopy yield similar results regarding hepatic fat quantification.
• Each sequence can be performed in one breath-hold.
• These sequences can be implemented in routine abdominal MRI protocols.
• Thus hepatic fat can be evaluated without relevant increase in scan time.




Similar content being viewed by others
Abbreviations
- BMI:
-
body mass index
- 95 % CI:
-
95 % confidence interval
- HISTO:
-
high-speed T2-corrected multi-echo single-voxel spectroscopy
- H MRS:
-
1H magnetic resonance spectroscopy
- ICC:
-
Intra-class correlation coefficient
- MRI:
-
Magnetic resonance imaging
- NAFLD:
-
non-alcoholic fatty liver disease
- NPV:
-
negative predictive value
- PPV:
-
positive predictive value
- PRESS:
-
point-resolved spectroscopy
- STEAM:
-
stimulated-echo acquisition mode
- VIBE:
-
volumetric interpolated breath-hold examination
- KORA:
-
Cooperative Health Research in the Region of Augsburg
References
Chalasani N, Younossi Z, Lavine JE et al (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 142:1592–1609
Cusi K (2009) Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 16:141–149
Lee JY, Kim KM, Lee SG et al (2007) Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol 47:239–244
Marcos A, Fisher RA, Ham JM et al (2000) Selection and outcome of living donors for adult to adult right lobe transplantation. Transplantation 69:2410–2415
Vernon G, Baranova A, Younossi ZM (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34:274–285
Williams CD, Stengel J, Asike MI et al (2011) Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140:124–131
Browning JD, Szczepaniak LS, Dobbins R et al (2004) Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40:1387–1395
Machado M, Marques-Vidal P, Cortez-Pinto H (2006) Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol 45:600–606
OECD (2014) Obesity update 2014. OECD, Paris. http://www.oecd.org/health/Obesity-Update-2014.pdf. Accessed 26 Oct 2014
Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F (2009) Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 51:433–445
Bohte AE, van Werven JR, Bipat S, Stoker J (2011) The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 21:87–97
Bashir MR, Merkle EM, Smith AD, Boll DT (2012) Hepatic MR imaging for in vivo differentiation of steatosis, iron deposition and combined storage disorder: single-ratio in/opposed phase analysis vs. dual-ratio Dixon discrimination. Eur J Radiol 81:e101–e109
Bashir MR, Zhong X, Dale BM, Gupta RT, Boll DT, Merkle EM (2013) Automated patient-tailored screening of the liver for diffuse steatosis and iron overload using MRI. AJR Am J Roentgenol 201:583–588
Zhong X, Nickel MD, Kannengiesser SA, Dale BM, Kiefer B, Bashir MR (2014) Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn Reson Med 72:1353–1365
Bashir MR, Zhong X, Nickel MD et al (2015) Quantification of hepatic steatosis with a multistep adaptive fitting MRI approach: prospective validation against MR spectroscopy. AJR Am J Roentgenol 204:297–306
Pineda N, Sharma P, Xu Q, Hu X, Vos M, Martin DR (2009) Measurement of hepatic lipid: high-speed T2-corrected multiecho acquisition at 1H MR spectroscopy–a rapid and accurate technique. Radiology 252:568–576
Fischer MA, Raptis DA, Montani M et al (2012) Liver fat quantification by dual-echo MR imaging outperforms traditional histopathological analysis. Acad Radiol 19:1208–1214
Tang A, Desai A, Hamilton G et al (2015) Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 274:416–425
Yokoo T, Bydder M, Hamilton G et al (2009) Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology 251:67–76
Meisamy S, Hines CD, Hamilton G et al (2011) Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 258:767–775
Tang A, Tan J, Sun M et al (2013) Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 267:422–431
Xiang QS, An L (1997) Water-fat imaging with direct phase encoding. J Magn Reson Imaging 7:1002–1015
Ma J (2004) Breath-hold water and fat imaging using a dual-echo two-point Dixon technique with an efficient and robust phase-correction algorithm. Magn Reson Med 52:415–419
Yu H, Reeder SB, Shimakawa A, Brittain JH, Pelc NJ (2005) Field map estimation with a region growing scheme for iterative 3-point water-fat decomposition. Magn Reson Med 54:1032–1039
DeFilippis AP, Blaha MJ, Martin SS et al (2013) Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 227:429–436
Baumeister SE, Volzke H, Marschall P et al (2008) Impact of fatty liver disease on health care utilization and costs in a general population: a 5-year observation. Gastroenterology 134:85–94
Koehler EM, Schouten JN, Hansen BE et al (2012) Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: results from the Rotterdam study. J Hepatol 57:1305–1311
German National Cohort (GNC) Consortium (2014) The German National Cohort: aims, study design and organization. Eur J Epidemiol 29:371–382
Henninger B, Zoller H, Rauch S et al (2015) Automated two-point Dixon screening for the evaluation of hepatic steatosis and siderosis: comparison with R2-relaxometry and chemical shift-based sequences. Eur Radiol 25:1356–1365
Kukuk GM, Hittatiya K, Sprinkart AM et al (2015) Comparison between modified Dixon MRI techniques, MR spectroscopic relaxometry, and different histologic quantification methods in the assessment of hepatic steatosis. Eur Radiol. doi:10.1007/s00330-015-3703-6
Acknowledgments
We thank Xiaodong Zhong and Radhouene Neji of Siemens Healthcare as principal authors of the prototype sequences and software packages.
The scientific guarantor of this publication is Holger Hetterich. The authors of this manuscript declare relationships with the following companies: Stephan A.R. Kannengießer is an employee of Siemens Healthcare. This study has received funding by The KORA research platform (Cooperative Research in the Region of Augsburg) and was initiated and financed by the Helmholtz Zentrum München - German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research and by the State of Bavaria. The KORA-MRI substudy received funding by the German Research Foundation (DFG, Deutsche Forschungsgemeinschaft). The KORA-MRI substudy was supported by an unrestricted research grant from Siemens Healthcare.
One of the authors has significant statistical expertise. Institutional review board approval was obtained. Written informed consent was obtained from all subjects in this study. Methodology: prospective, cross sectional, diagnostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hetterich, H., Bayerl, C., Peters, A. et al. Feasibility of a three-step magnetic resonance imaging approach for the assessment of hepatic steatosis in an asymptomatic study population. Eur Radiol 26, 1895–1904 (2016). https://doi.org/10.1007/s00330-015-3966-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3966-y