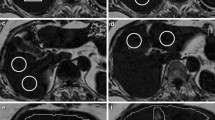
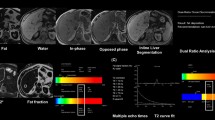
Abstract
This study associated the liver proton density fat fraction (PDFF), measured by multi-echo Dixon (ME-Dixon) and breath-hold single-voxel high-speed T2-corrected multi-echo 1H magnetic resonance spectroscopy (HISTO) at 1.5 T, with serum biomarkers and liver fibrosis stages. This prospective study enrolled 75 patients suspected of liver fibrosis and scheduled for liver biopsy and 23 healthy participants with normal liver function. The participant underwent ME-Dixon and HISTO scanning. The agreement of PDFF measured by ME-Dixon (PDFF-D) and HISTO (PDFF-H) were compared. Correlations between PDFF and serum fat biomarkers (total cholesterol, triglyceride, and high- and low-density lipoproteins) and the liver fibrosis stages were assessed. PDFF were compared among the liver fibrosis stages (F0–F4) based on clinical liver biopsies. The Bland–Altman plot showed agreement between PDFF-D and PDFF-H(LoA, − 4.44 to 6.75), which have high consistency (ICC 0.752, P < 0.001). The correlations with the blood serum markers were mild to moderate (PDFF-H: r = 0.261–0.410, P < 0.01; PDFF-D: r = 0.265–0.367, P < 0.01). PDFF-D, PDFF-H, and steatosis were distributed similarly among the liver fibrosis stages. PDFF-H showed a slight negative correlation with the liver fibrosis stages (r = − 0.220, P = 0.04). Both ME-Dixon and HISTO sequences measured liver fat content noninvasively. Liver fat content was not directly associated with liver fibrosis stages.
Similar content being viewed by others
Introduction
Liver fibrosis is defined as excess deposition of extracellular matrix components such as collagens, glycoproteins, and proteoglycans in the liver. Liver fibrosis is a complex pathological process accompanied by steatosis, inflammation, and other changes. Liver fibrosis, a public health problem, is closely associated with various prevalent causes of chronic liver damage1. This response to liver damage is potentially reversible, so early diagnosis and accurate staging are highly important in clinical practice2, 3.
Liver steatosis is a pathological condition characterized by excessive fat deposition in the hepatocytes. It is the most common chronic liver disease, seen in viral hepatitis, genetic lipodystrophies, alcohol abuse, and more. If it has no competing causes, it is called nonalcoholic fatty liver disease (NAFLD)4, which can progress to more aggressive forms such as hepatitis, fibrosis, and cirrhosis5.
Recently, fast quantitative MRI techniques for acquiring proton density fat fraction maps within one breath-hold cycle have been introduced. Multi-echo Dixon (ME-Dixon) is a variant of 3D multi-gradient-echo sequences that allows fast imaging for simultaneous assessment of the fat fraction and transverse-relaxation time. It is a confounder-corrected chemical shift-encoded MRI technique that could potentially quantify total liver fat content. A breath-hold single-voxel high-speed T2-corrected multi-echo 1H MRS (HISTO) sequence can quantify lipid percentages by correcting for the R2 relaxations of water and fat. It is more accurate than traditional MRS in quantifying fat content. Recent studies have shown that these techniques could provide an accurate quantification of liver fat, verified by liver biopsies6, 7.
Liver fibrosis and steatosis are two independent but closely related pathological processes. Some research3, 8, 9 had pointed out the effect of steatosis on liver fibrosis. However, few studies have focused on the impact of liver fat content on the liver fibrosis stages. Therefore, this prospective study aimed to explore the association between liver fat content, assessed by the ME-Dixon and HISTO techniques, and the liver fibrosis stages.
Results
The Bland–Altman plot for the PDFF agreement is shown in Fig. 1. The mean difference (bias) between PDFF-D and PDFF-H was 1.15%, and the limits of agreement were − 4.44 to 6.75%. Some points were outside the 95% limits of the agreement. ICC showed a good consistency (ICC 0.752, P < 0.01) between PDFF-D and PDFF-H.
Bland–Altman plot for proton density fat fraction (PDFF), estimated using the breath-hold single-voxel high-speed T2-corrected multi-echo 1H MRS (HISTO) and multi-echo Dixon (ME-Dixon) sequences. The plot shows the − 1.96 and + 1.96 SD limits of the agreement (dotted lines) around the mean difference. PDFF-D PDFF estimated by ME-Dixon, PDFF-H PDFF estimated by HISTO.
The relationships between PDFF-D and PDFF-H and the blood serum parameters are displayed as a scatter plot matrix in Fig. 2. The best indicators were those separating the clusters in an optimal way, with no overlapping in the scatter plot.
A scatterplot matrix with linear regression, associating the fat content measured by the breath-hold single-voxel high-speed T2-corrected multi-echo 1H MRS (HISTO) and multi-echo Dixon (ME-Dixon) sequences with the blood serum parameters. PDFF-D proton density fat fraction of ME-Dixon, DPDFF-H proton density fat fraction of HISTO, FIB-4 Fibrosis-4 index, TCHO total cholesterol, TG triglyceride, HDL high-density lipoproteins, LDL low-density lipoproteins.
Table 1 shows mild-to-moderate correlations between PDFF and the blood serum parameters (total cholesterol, triglyceride, and high- and low-density lipoproteins), somewhat higher in PDFF-H than in PDFF-D.
The PDFF and steatosis distributions for the healthy volunteers and patients in the various fibrosis stages are shown in Table 2. Both showed similar steatosis distribution among the various fibrosis stages. The Kruskal–Wallis test found no difference among the fibrosis stage groups (Fig. 3). The correlations between PDFF and the fibrosis stage (S) and inflammation activity (G) in the biopsies are shown in Table 3. A mild negative correlation was found between PDFF-H and the fibrosis stages (r = − 0.220, P = 0.04).
Kruskal–Wallis test to assess the difference between the fibrosis stage groups. Outlier data points marked by open circles were 1.5–3.0 interquartile ranges from the box ends. Outlier data points marked by asterisks were more than three interquartile ranges from the box ends. PDFF proton density fat fraction, HISTO breath-hold single-voxel high-speed T2-corrected multi-echo 1H MRS, ME-Dixon multi-echo Dixon, PDFF-D PDFF estimated by ME-Dixon, PDFF-H PDFF estimated by HISTO.
Discussion
NAFLD, a common liver steatosis condition, is a complex disease spectrum, ranging from steatosis to non-alcoholic steatohepatitis and fibrosis10. Significant fibrosis is an important histological feature of non-alcoholic steatohepatitis, an aggressive form of this disease11. Therefore, liver fat content and fibrosis are closely associated and important for the disease prognosis.
Liver biopsy is currently the gold standard method to assess liver fibrosis and fat content in clinical practice. However, it is invasive and associated with sampling and interpretation errors, potential complications, and poor patient acceptance, restricting its wide clinical application12, 13. Noninvasive methods, especially quantitative imaging ones, receive increasing attention and use in clinical monitoring of liver fat content14 and liver fibrosis staging15,16,17.
This study investigated two MRI-based methods to quantify liver fat content and probe its impact on liver fibrosis staging.
Quantification of liver fat with ME-Dixon and HISTO sequences
PDFF is a fundamental property of tissues, reflecting their fat concentration. PDFF is increasingly recognized as an accurate, noninvasive liver fat detection and quantification method18.
ME-Dixon and HISTO sequences are frequently used to quantify liver fat content in general practice. ME-Dixon sequence is a variant of the modified chemical shift-encoded MRI method, which provides accurate lipid fraction and, thus, fat content quantification in the entire liver. HISTO sequence is a fast breath-hold MRS method for T2-corrected liver lipid measurement. It overcomes the accuracy and speed limitations of the conventional 1H MRS. Both ME-Dixon and HISTO sequences allow for acquisition within a single breath-hold cycle.
Both PDFF-D and PDFF-H showed significant correlation with TCHO, TG and LDL. Which provide the potential value as biomarker of liver fat content in clinical medicine. Like some previous studies19,20,21,22,23, we observed a significant correlation between PDFF-D and PDFF-H (ICC 0.752, P < 0.01) even though the Bland–Altman plot showed a limit to the agreement between PDFF-D and PDFF-H, and ICC showed a good consistent between them. There could be several reasons for our correlation result being weaker than the strong correlation (r = 0.984 and 0.986) observed between chemical shift-encoded-PDFF and MRS-PDFF in previous studies20, 22. First, HISTO is a single-voxel spectroscopy acquisition mode, obtained in this study using a VOI placed in the right posterior liver segment. However, it is known that fat distribution in the liver is often heterogeneous. Consequently, sampling errors may have impacted our HISTO results. Second, 63 of the 75 patients in our study had a liver fat content lower than 5%. Pineda et al.24 reported that the signal-to-noise ratio decreases with the decrease in the liver fat content. This may have affected our ability to accurately quantify the liver fat content by the ME-Dixon and HISTO sequences.
The similar results were also seen in the research of Zhong et al.25 and Bashir et al.26. They pointed that MRI-based measure of liver PDFF are clinically feasible and accurate. The difference is that they both based on 3.0 T scan, while our study was based on 1.5 T scan. These maybe a testament of MRI-based measurements of PDFF across a variety of platforms.
The impact of liver fat on liver fibrosis staging
Liver fibrosis is an early stage of liver damage. It is a common pathological process in many chronic liver diseases. The initial stage is chronic liver inflammation, followed by diffuse liver fibrosis, replacement of the normal liver architecture by regenerative hepatic nodules, and eventually liver failure27. Liver fibrosis is the leading long-term outcome predictor among all the histopathological changes identified in chronic liver diseases28. Therefore, noninvasive liver fibrosis assessment has always been the focus of clinical research. Liver fibrosis staging is influenced by complex histopathological changes such as steatosis and inflammation. The relationship between steatosis and fibrosis has attracted our attention. We defined steatosis as PDFF > 5%, and so also PDFF-D and PDFF-H > 5%. Nevertheless, we found no association between the various fibrosis stage groups and PDFF-D, PDFF-H, or steatosis. Spearman correlation showed that PDFF-H was weakly and negatively correlated with the fibrosis stages (r = − 0.220, P = 0.04). All these may suggest that the liver fat content has no direct effect on liver fibrosis stage similar with the previous study29. It was different from Ajmera et al.30, their research suggested a clear link between steatosis and fibrosis severity. The reasons for this may because: first, in their study, the number of patients with lower and higher liver fat content was similar. While, in our study most participants had low liver fat content. Second, the subject of their research was NAFLD, but our study patient population consist a large extent of HBV. Further studies will be needed to validate these findings.
We found through a literature review that a “two-hit” hypothesis was established as early as in 199831. Steatosis is just the first “hit” on the liver. Whether patients with steatosis progress to necroinflammation or fibrosis depends on the presence of some other factors, known as the second “hit.” This could be a plausible endorsement for our findings. This ‘two-hit’ hypothesis has further evolved into the ‘‘multiple-hit” hypothesis based on novel findings from recent research32. In addition to steatosis, other factors, including insulin resistance, inflammatory mediators, lipopolysaccharides, and genetic factors, drive the liver parenchyma toward fibrosis.
Limitations
This study had some limitations. First, we did not compare the MRI-based PDFF with semi-automatic histological fat quantification. Liver biopsies are usually done at our hospital to determine the etiology, so the pathologists ignore the fat content. Furthermore, liver biopsies have inherent sampling errors, especially when assessing liver fat content. Second, we did not divide the patients based on the etiology. There are five causes in our patients in this research (HBV, NAFLD, chronic liver disease without hepatitis virus infection, drug toxicities, and autoimmune). We found that in group of NAFLD, the PDFF was higher than other groups’ (P < 0.05), the difference was statistically significant, and the difference between other groups has no significant. This may have added to the confounding factors in this study and should be improved in the future. Third, as a single-voxel protocol, HISTO should be sampled in multiple sites, averaging the results. Such an approach could solve the sampling error issue and improve the accuracy; however, we sampled only one site in this study. One side, the single sampling method was used to increase the comparability of these two methods. And they were placed separately at the scanning by the same physician. On the other hand, the manual operation was really difficult to ensure the absolute consistency of the two mining positions. This may affect the consistency of the two methods. In future clinical application, multi-point sampling is still recommended.
Future work is needed to assess a larger number of patients in one etiology and to correlate PDFF with histological findings. For better comparison with ME-Dixon, we only chose one sample site of HISTO. Applied as a fat quantification method to the clinic, we recommend multiple sites of HISTO.
Methods
Study design and patients
This prospective study was conducted in accordance with the guidelines of the Declaration of Helsinki for Human Research and approved by the ethics committee of Lanzhou University Second Hospital (2020A-236), and all participants provided written informed consent.
This study enrolled 98 patients with chronic liver diseases and 24 healthy adult volunteers without serious health problems from Oct 2020 to June 2022. The inclusion criterion for the patients was adults with a chronic liver disease scheduled to undergo a liver biopsy. The inclusion criterion for the healthy volunteers was adults without serious health problems. The exclusion criteria included contraindications to MRI (claustrophobia, metal implants, or pacemakers). Figure 4 summarizes the participant recruitment process for the study. The underlying causes of the chronic liver disease in the 75 patients were hepatitis B (n = 56), nonalcoholic steatohepatitis (n = 8), a chronic liver disease without hepatitis virus infection (n = 6), drug toxicities (n = 4), and autoimmune (n = 1). Liver fibrosis was confirmed by biopsy in 64 patients. The liver biopsy and MRI were performed within a one-week interval.
MRI examination
MRI examination was performed on a 1.5 T MR scanner (MAGNETOM Aera, Siemens Healthineers, Erlangen, Germany) using a combination of 18-channel body and 32-channel spine matrix coil elements. The participants underwent MR scanning using the ME-Dixon and HISTO protocols in the LiverLab package (Siemens Healthineers). An axial ME-Dixon sequence was performed with the following parameters: TR = 15.6 ms; TE = 2.38, 4.76, 7.14, 9.52, 11.9, and 14.28 ms, flip angle = 4°, FOV = 450 × 394 mm; slice thickness = 3.5 mm, voxel size = 1.4 × 1.4 × 3.5 mm, number of slices = 64. The HISTO sequence was performed with the following parameters: TR = 3,000 ms, TE = 12, 24, 36, 48, and 72 ms, flip angle = 90°, VOI = 30 × 30 × 30 mm, scan time is 15 s. We selected the right liver lobe (hilar level) near the ME-Dixon sequence ROI for the HISTO sequence, avoiding bile ducts and blood vessels. Figure 5 shows an example of the ME-Dixon and HISTO sequences. Subsequently, we calculated the proton density fat fraction (PDFF), defined as the fat-to-total (fat and water) signal strength ratio33, and named PDFF-D and PDFF-H, respectively.
An example of the ME-Dixon and HISTO sequences. Multi-echo Dixon (ME-Dixon) and breath-hold single-voxel high-speed T2-corrected multi-echo 1H MRS (HISTO) sequence images of a 37-year-old female patient with chronic hepatitis B and G2S2 liver fibrosis in histology. The HISTO and ME-Dixon sequence reports show a proton density fat fraction (PDFF) of 5.0 and 5.6%, respectively. D is the slice corresponding to the report in C, and the red squares indicate the placement of the region of interest.
Biochemical tests and liver biopsies
One clinical medicine expert reviewed the patients’ medical records. Blood serum parameters were recorded within one week before or after the MRI examination. The participants’ demographic and clinical characteristics are presented in Table 4. The Fibrosis-4 index was calculated based on clinical and routine laboratory variables using previously-defined algorithms34. Histopathologic assessments were performed by an experienced pathologist, who graded liver fibrosis (F0–F4) based on the Scheuer semiquantitative scoring system35.
Statistical analysis
Agreement between fat content calculated from the ME-Dixon and HISTO sequences and blood serum parameters was assessed in three steps. First, we estimated the agreement using Bland–Altman plots, in which the difference between PDFF-D and PDFF-H was plotted against their means with their 95% limits of agreement. Subsequently, the intraclass correlation coefficient (ICC) assessed the degree of agreement between PDFF-D and PDFF-H. Second, we drew a scatterplot and performed linear regression to assess the association between the PDFF-D or PDFF-H and the blood serum parameters. Third, we used Spearman correlation coefficients to characterize the association of PDFF-D or PDFF-H measures and the blood serum parameters.
The Kruskal–Wallis test evaluated the distribution of PDFF-D and PDFF-H among the liver fibrosis stages. Fisher’s exact probabilities tested steatosis distribution among the liver fibrosis stages. PDFF-D and PDFF-H were defined as steatosis when > 5%. The Spearman’s rank correlation coefficients assessed the correlation between PDFF and the fibrosis stages.
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA), and R statistical software version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Two-sided P values < 0.05 were considered statistically significant.
Conclusions
ME-Dixon and HISTO sequences are noninvasive MR imaging methods for measuring liver fat content. The liver fat content showed no direct impact on the liver fibrosis stages.
Data availability
Availability of data and materials. All data generated and analyzed during the current study will be available from the corresponding author on reasonable request.
References
Pinzani, M. Hepatic fibrosis: A global clinical problem. In Stellate Cells in Health and Disease (eds Gandhi, C. R. & Pinzani, M.) 29–39 (Academic Press, 2015).
Chinese Society of Hepatology CMaCSOG, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and therapy of hepatic fibrosis in 2019. Chin. J. Hepatol. 27, 657–668 (2019).
Li, C., Li, R. & Zhang, W. Progress in non-invasive detection of liver fibrosis. Cancer Biol. Med. 15, 124–136 (2018).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Benedict, M. & Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 9, 715–732 (2017).
An Tang, M. et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 267, 422–431 (2013).
Ilkay, S. et al. Hepatic steatosis: Quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 267, 767–775 (2013).
Yoon, H., Shin, H. J., Kim, M. J. & Lee, M. J. Quantitative imaging in pediatric hepatobiliary disease. Korean J. Radiol. 20, 1342–1357 (2019).
Wáng, Y. X. J. et al. A combined use of intravoxel incoherent motion MRI parameters can differentiate early-stage hepatitis-b fibrotic livers from healthy livers. SLAS Technol. 23, 259–268 (2018).
Pavlides, M. et al. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int. 37, 1065–1073 (2017).
Angulo, P. et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397 (2015).
Goodman, Z. D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 47, 598–607 (2007).
West, J. & Card, T. J. G. Reduced mortality rates following elective percutaneous liver biopsies. Gastroenterology 139, 1230–1237 (2010).
Starekova, J. & Reeder, S. B. Liver fat quantification: Where do we stand?. Abdom. Radiol. (NY) 45, 3386–3399 (2020).
Stoopen-Rometti, M. et al. Diagnosis and quantification of fibrosis, steatosis, and hepatic siderosis through multiparametric magnetic resonance imaging. Rev. Gastroenterol. Mex. 82, 32–45 (2017).
Kupczyk, P. A. et al. Quantitative MRI of the liver: Evaluation of extracellular volume fraction and other quantitative parameters in comparison to MR elastography for the assessment of hepatopathy. Magn. Reson. Imaging 77, 7–13 (2021).
Dennis, A. et al. A composite biomarker using multiparametric magnetic resonance imaging and blood analytes accurately identifies patients with non-alcoholic steatohepatitis and significant fibrosis. Sci. Rep. 10, 15308 (2020).
Yokoo, T. et al. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using mr imaging: A meta-analysis. Radiology 286, 486–498 (2018).
Boris Guiu, M. et al. Quantification of liver fat content: Comparison of triple-echo chemical shift gradient-echo imaging and in vivo proton MR spectroscopy. Radiology 250, 95–102 (2009).
Idilman, I. S. et al. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol. 57, 271–278 (2016).
Kukuk, G. M. et al. Comparison between modified Dixon MRI techniques, MR spectroscopic relaxometry, and different histologic quantification methods in the assessment of hepatic steatosis. Eur. Radiol. 25, 2869–2879 (2015).
Hu, H. H., Kim, H. W., Nayak, K. S. & Goran, M. I. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity (Silver Spring) 18, 841–847 (2010).
Wu, C. H. et al. Quantification of hepatic steatosis: A comparison of the accuracy among multiple magnetic resonance techniques. J. Gastroenterol. Hepatol. 29, 807–813 (2014).
Nashiely Pineda, P. et al. Measurement of hepatic lipid: High-speed T2-corrected multiecho acquisition at 1H MR spectroscopy—A rapid and accurate technique1. Radiology 252, 568–576 (2009).
Zhong, X. et al. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn. Reson. Med. 72, 1353–1365 (2014).
Bashir, M. R. et al. Quantification of hepatic steatosis with a multistep adaptive fitting MRI approach: Prospective validation against MR spectroscopy. AJR Am. J. Roentgenol. 204, 297–306 (2015).
Ginès, P. et al. Liver cirrhosis. Lancet 398, 1359–1376 (2021).
Serra-Burriel, M. et al. Transient elastography for screening of liver fibrosis: Cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J. Hepatol. 71, 1141–1151 (2019).
Hayashi, T. et al. Hepatic fat quantification using the two-point Dixon method and fat color maps based on non-alcoholic fatty liver disease activity score. Hepatol. Res. 47, 455–464 (2017).
Ajmera, V. et al. Magnetic resonance imaging proton density fat fraction associates with progression of fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 155, 307–310 (2018).
Cp, D. & Ofw, J. Steatohepatitis: A tale of two “Hits”?. Gastroenterology 114, 842–845 (1998).
Kumar, S., Duan, Q., Wu, R., Harris, E. N. & Su, Q. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Adv. Drug Deliv. Rev. 176, 113869. https://doi.org/10.1016/j.addr.2021.113869 (2021).
Reeder, S. B., Hu, H. H. & Sirlin, C. B. Proton density fat-fraction: A standardized MR-based biomarker of tissue fat concentration. J. Magn. Reson. Imaging 36, 1011–1014 (2012).
Sterling, R. K. et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43, 1317–1325 (2006).
Desmet, V. J., Gerber, M., Hoofnagle, J. H., Manns, M. & Scheuer, P. J. Classification of chronic hepatitis diagnosis grading and staging. Hepatology 19, 8 (1994).
Acknowledgements
The authors acknowledge the support of Siemens Healthineers, which provides research support to the Lanzhou University Second Hospital.
Funding
The Cuiying Scientific and Technological Innovation Program of' Lanzhou University Second Hospital (Grant no. CY2021-QN-B09), the Science and Technology Project of Gansu Province (Grant no. 21JR11RA122) and Department of Education of Gansu Province: Innovation Fund Project (Grant no. 2022B-056).
Author information
Authors and Affiliations
Contributions
Y.J.: Conceptualization, data curation, investigation, formal analysis, visualization, writing—original draft. J.Z.: Conceptualization, data curation, investigation, writing—review and editing. F.F.: Conceptualization, methodology, software, writing—review and editing. P.Y.: Data curation, software. L.M.: Conceptualization, software. T.G.: Conceptualization, software. S.W.: Conceptualization, software, writing—review and editing, supervision. J.Z.: Conceptualization, investigation, funding acquisition, project administration, resources, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors Yanli Jiang, Jie Zou, Fengxian Fan, Pin Yang, Laiyang Ma, Tiejun Gan, Jing Zhang declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The author Shaoyu Wang who is from Siemens Healthineers also declare that he have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, Y., Zou, J., Fan, F. et al. Application of multi-echo Dixon and MRS in quantifying hepatic fat content and staging liver fibrosis. Sci Rep 13, 12555 (2023). https://doi.org/10.1038/s41598-023-39361-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39361-6
- Springer Nature Limited