Abstract
Purpose
To identify magnetic resonance (MR)/MR cholangiopancreatography (MRCP) imaging signs helpful in the differential diagnosis between serous cystadenomas (SCAs) and mucinous cystic neoplasms (MCNs), arising from the body/tail of the pancreas.
Material and methods
This retrospective study had institutional review board approval and informed consent was waived. Fifty-three patients with non-communicating cystic pancreatic neoplasm of the body/tail, undergoing MR/MRCP, were included. Qualitative image analysis assessed the macroscopic pattern, number of cysts, presence of central scar, contrast enhancement of peripheral wall, and mural nodules. Quantitative analysis assessed the maximum diameter of the neoplasm, thickness of the peripheral wall, and calibre of the upstream main pancreatic duct.
Results
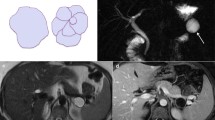
Histopathology results revealed that 27/53 (51 %) were SCAs, 26/53 (49 %) were MCNs. Microcystic pattern was observed in 88.2 % of SCAs and 11.8 % of MCNs; macrocystic pattern was observed in 90.5 % of MCNs and 9.5 % of SCAs (p < 0.0001). Central scar was detected in 29.6 % of SCAs and no MCNs (p = 0.003). Contrast enhancement of the peripheral wall was evident in 99.5 % of MCNs and 11.5 % of SCAs (p < 0.0001); mural nodules were depicted in 94.1 % of MCNs and 5.9 % of SCAs (p < 0.0001).
Median maximum diameter was 54 mm for MCNs, 32 mm for SCAs (p = 0.001); median wall thickness was 4 mm for MCNs, 2 mm for SCAs (p < 0.0001).
Conclusions
Macrocystic pattern, enhancement of a peripheral wall and mural nodules are suggestive of MCNs; whereas microcystic pattern, lack of peripheral wall and central scar are suggestive of SCAs.
Key Points
• MCNs have macrocystic patterns, contrast enhancement of the peripheral wall and mural nodules
• Microcystic pattern and central scar are suggestive of SCA
• Mural nodules detected in MCNs correlate with epithelial dysplasia
• Chronic obstructive pancreatitis is equally depicted in patients with MCNs and SCAs






Similar content being viewed by others
References
Basturk O, Coban I, Adsay NV (2009) Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med 133:423–438
Compagno J, Oertel JE (1978) Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol 69:573–580
Tanaka M, Chari S, Adsay V et al (2006) International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 6:17–32
Zamboni G, Fukushima N, Hruban R, Klöppel G (2010) Mucinous cystic neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND (eds) WHO classification of tumours of the digestive system. IARC, Lyon, pp 300–303
Zamboni G, Scarpa A, Bogina G et al (1999) Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 23:410–422
Thompson LD, Becker RC, Przygodzki RM, Adair CF, Heffess CS (1999) Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: a clinicopathologic study of 130 cases. Am J Surg Pathol 23:1–16
Crippa S, Salvia R, Warshaw AL et al (2008) Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg 247:571–579
Fritz S, Warshaw AL, Thayer SP (2009) Management of mucin-producing cystic neoplasms of the pancreas. Oncologist 14:125–136
Gaujoux S, Brennan MF, Gonen M et al (2011) Cystic lesions of the pancreas: changes in the presentation and management of 1,424 patients at a single institution over a 15-year time period. J Am Coll Surg 212:590–600, discussion 600–593
Bassi C, Salvia R, Molinari E, Biasutti C, Falconi M, Pederzoli P (2003) Management of 100 consecutive cases of pancreatic serous cystadenoma: wait for symptoms and see at imaging or vice versa? World J Surg 27:319–323
Manfredi R, Bonatti M, D'Onofrio M et al (2012) Incidentally discovered benign pancreatic cystic neoplasms not communicating with the ductal system: MR/MRCP imaging appearance and evolution. Radiol Med. doi:10.1007/s11547-012-0837-3
Crippa S, Fernandez-Del Castillo C, Salvia R et al (2010) Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol 8:213–219
Manfredi R, Graziani R, Motton M et al (2009) Main pancreatic duct intraductal papillary mucinous neoplasms: accuracy of MR imaging in differentiation between benign and malignant tumors compared with histopathologic analysis. Radiology 253:106–115
Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF (2005) Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics 25:1471–1484
Salvia R, Partelli S, Crippa S et al (2009) Intraductal papillary mucinous neoplasms of the pancreas with multifocal involvement of branch ducts. Am J Surg 198:709–714
Berland LL, Silverman SG, Gore RM et al (2010) Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 7:754–773
Lee KS, Sekhar A, Rofsky NM, Pedrosa I (2010) Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 105:2079–2084
Kalb B, Sarmiento JM, Kooby DA, Adsay NV, Martin DR (2009) MR imaging of cystic lesions of the pancreas. Radiographics 29:1749–1765
Reddy RP, Smyrk TC, Zapiach M et al (2004) Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol 2:1026–1031
Andrejevic-Blant S, Kosmahl M, Sipos B, Kloppel G (2007) Pancreatic intraductal papillary-mucinous neoplasms: a new and evolving entity. Virchows Arch 451:863–869
Kosmahl M, Pauser U, Peters K et al (2004) Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch 445:168–178
Griffin N, Charles-Edwards G, Grant LA (2012) Magnetic resonance cholangiopancreatography: the ABC of MRCP. Insights Imaging 3:11–21
Delavaud C, d’Assignies G, Cros J et al (2014) CT and MR imaging of multilocular acinar cell cystadenoma: comparison with branch duct intraductal papillary mucinous neoplasia (IPMNs). Eur Radiol. doi:10.1007/s00330-014-3248-0
Manfredi R, Bonatti M, Mantovani W et al (2013) Non-hyperfunctioning neuroendocrine tumours of the pancreas: MR imaging appearance and correlation with their biological behaviour. Eur Radiol 23:3029–3039
Wu J, Jiao Y, Dal Molin M et al (2011) Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 108:21188–21193
Acknowledgements
The scientific guarantor of this publication is Roberto Pozzi Mucelli. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional review board approval was obtained. Written informed consent was waived by the institutional review board. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manfredi, R., Ventriglia, A., Mantovani, W. et al. Mucinous cystic neoplasms and serous cystadenomas arising in the body-tail of the pancreas: MR imaging characterization. Eur Radiol 25, 940–949 (2015). https://doi.org/10.1007/s00330-014-3493-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3493-2