Abstract
Warming over Antarctica is leading to changes in the zooplankton communities inhabiting the Southern Ocean. It has been observed that zooplankton not only regulates phytoplankton through grazing, but also through the recycling of nutrients that are essential for phytoplankton growth. In this way, the effects of warming on zooplankton populations will change the amount or proportion at which recycled nutrients are restored. To estimate how the recycled nutrients released by zooplankton populations, dominated by krill (Euphausia superba), amphipods or copepods, affect the phytoplankton uptake and communities, we performed four incubation experiments: two close to the Antarctic Peninsula and two at the Southern Atlantic Ocean. Our results showed a stimulating effect of the addition of metabolites on ammonia removal rates and on the net growth of phytoplankton communities, with different responses amongst the different phytoplankton groups. According to our results, phytoplankton net growth and community composition may be altered if this relevant source of nutrients is lost due to projected changes in the abundance or distribution of these zooplankton populations.
Similar content being viewed by others
Introduction
One of the main characteristics of the Southern Ocean is the low phytoplankton biomass present in these high nutrient water masses (e.g., El-Sayed 1984), although the limiting factors that control phytoplankton growth (such as temperature or light availability) are occasionally relieved and lead to large phytoplankton blooms (Sakshaug and Holm-Hansen 1984).
Antarctic coastal waters are likely to be nutrient rich and iron sufficient (Sañudo-Wilhelmy et al. 2002) in contrast with lower iron availability in open waters (Sarthou et al. 2011). Thus, chlorophyll a concentrations are usually higher in coastal waters near the Antarctic Peninsula when compared with oceanic waters offshore (Marrari et al. 2008).
The phytoplankton community in the Southern Ocean is mainly composed by diatoms and dinoflagellates (Fiala et al. 1998; Garibotti et al. 2005; Kopczyńska et al. 2007). Amongst these, diatoms are significant contributors to total phytoplankton biomass in polar communities, including genera such as Thalassiosira, Nitzschia, Chaetoceros, Corethron, Odontella and Rhizosolenia (e.g., Clarke and Leakey 1996; Arrigo et al. 1999), and also play a key role during spring blooms (Sakshaug and Holm-Hansen 1984). In this context of high nutrient concentrations, Antarctic phytoplankton usually shows low specific nitrate uptake rates, contrasting with the high utilization of available ammonium (Dugdale and Wilkerson 1991) that reflects a high dependence on ammonium supply (Agustí and Duarte 2000).
This form of reduced nitrogen in Antarctic waters can be obtained from several sources: sea ice, that usually represents a source of reduced organic and inorganic nitrogen during the melting season (e.g., Brandini and Bauman 1997), microbial regeneration (Goeyens et al. 1991), and, in some coastal areas, terrestrial sources from bird and mammal colonies (Tatur and Myrcha 1983). Amongst ammonia sources, zooplankton recycling of nutrients also plays an important role, conditioning the pelagic ecosystem in the Southern Ocean to maintain it at a high productivity level (Alcaraz et al. 1998; Lehette et al. 2012; Arístegui et al. 2014).
The Antarctic zooplankton community includes, amongst other groups, copepods, amphipods, and salps and is often dominated by krill (Euphausia superba), the central node linking primary producers, and higher trophic levels in the Antarctic marine food web (Murphy et al. 2007; Santana et al. 2013).
Zooplankton not only regulates phytoplankton populations through predation, but also through the efficient recycling of macro- and micronutrients (i.e., nitrogen, phosphorus, and iron) that are essential for phytoplankton growth (Sterner 1986; Vanni 2002; Tovar-Sánchez et al. 2007). Amongst all the recyclable nutrients, ammonium (NH4 +) is the principal nitrogenous excretion product of zooplankton (Corner and Davies 1971) and may represent the major portion of the total nitrogenous nutrient load assimilated in daily primary production (Biggs 1982). In fact, ammonia has been previously described as a preferred nitrogen source for phytoplankton growth (i.e., Probyn and Painting 1985; Alcaraz et al. 1998). Despite the typically low NH4 + concentrations in the Southern Ocean (Priddle et al. 1997), ammonia supply has been experimentally shown to trigger blooms in mesocosms deployed in Antarctica (Agustí and Duarte 2000; Agustí et al. 2009).
Previous observations on krill abundance and distribution (Loeb et al. 1997; Atkinson et al. 2004) have shown that warming over Antarctica is leading to changes in these zooplankton communities, caused by interannual changes in the extent of sea ice in areas crucial to krill recruitment (Smetacek 2008). Increased temperatures also influence the geographical distribution and abundance of other Antarctic macrozooplankton groups as salps (Atkinson et al. 2004; Mackey et al. 2012; Steinberg et al. 2015). In fact, while the current population of krill in the Southern Ocean may be only 20% of its pre-1980s size (Atkinson et al. 2004), the highest salp densities have occurred (Atkinson et al. 2004).
Changes in zooplankton abundances and species composition may have consequences for phytoplankton populations through the alteration of predation rates and nutrient recycling. Due to the major role that krill organisms play in the Antarctic ecosystem (Loeb et al. 1997), the decline in their standing stock may have serious effects in their contribution to recycled ammonia and in the Southern Ocean food web.
In a future ocean, with altered zooplankton communities, the input of zooplankton-recycled nutrients may change, with consequences for the inhabiting phytoplankton communities.
To clarify how phytoplankton populations respond to recycled nutrients and elucidate the consequences of future altered recycling, here, we performed four microcosm experiments. These were conducted south and north of the Antarctic Convergence, and involved the addition of excretion products of the in situ zooplankton community (in our experiments, krill, amphipods, and copepods). We assessed the nutrient consumption along each incubation and the responses of the phytoplankton communities to excreted nutrient additions.
Materials and methods
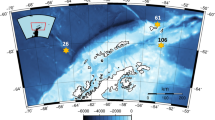
Four experiments were conducted both sides of the Antarctic Convergence: next to the Antarctic Peninsula, one experiment took place during ICEPOS 2 (2005) and one during ATOS II (2009). In the southern Atlantic Ocean, we performed two experiments during the cruise LOHAFEX (2009) (Fig. 1).
Zooplankton communities were sampled in vertical tows reaching 200 m deep, using a WP-2 net, in the LOHAFEX cruise, where communities were dominated by amphipods (experiment 1) and copepods (experiment 2) (Table 1). During the cruises ICEPOS 2 and ATOS II, krill swarms were located using a Simrad™ EK60 multifrequency echosounder. Then, an IKMT net with 1 cm mesh size was deployed and trawled at three knots for 20 min to sample those water layers with the highest krill abundance, specifically 40–70 m during ICEPOS 2 and 20–30 m deep during ATOS II. A sufficient number of zooplankton specimens (approximately 30–50 krill individuals in ICEPOS 2 and ATOS II, and up to 300 amphipods or the whole zooplankton community in LOHAFEX experiments) extracted from natural healthy zooplankton communities (Table 1) were directly transferred to buckets with 0.2 µm filtered surface seawater and allowed to excrete for 48 h while checking the increase in ammonia concentration daily (see in the following). When concentrations increased up to 50–100 µmol NH4 L−1, the water where zooplankton had been excreting was filtered through GF/F filters, and considered as the solution containing excretion products, or metabolite solution (MS).
Unfiltered surface water (5 m) was sampled with Niskin bottles mounted onto a Rosette sampling system and inoculated with enough MS solution to raise NH4 concentrations to about 5 µmol NH4 L−1. After mixing, water was distributed into four 2 L replicate Teflon bags (or two replicate 2 L quartz bottles, in ICEPOS 2 experiment), both systems allowing all the solar radiation to pass through. The same number of Teflon bags or quartz bottles was also filled with unfiltered 5 m surface water, but without MS solution, as control replicates. These containers were completely filled to avoid bubbles, and were enclosed in large mesh nets anchored at the bottom of the incubation tank, to prevent them from floating at the surface. In addition, after every sampling, Teflon bags were carefully manipulated and closed, allowing trapped air to escape. The experiments were performed on deck of the R/V Hespérides (during ICEPOS 2 and ATOS II) and Polarstern (LOHAFEX) in a place free of shade, receiving total incident solar radiation. Incubation tanks were fed with running surface seawater systems (3–5 m depth), fast and efficient to maintain the incubators at in situ (±1 °C) temperature conditions. The four experiments took place during summer, at close dates during February–March. Incubations lasted from 6 days (ATOS II) up to 13 days (LOHAFEX 1) (Table 1). Metal free techniques were used in all manipulations during our experiments.
50 mL of experimental water were sampled every second day and filtered into Whatmann GF/F filters to measure chlorophyll a concentration, following the fluorometric method described by Parsons et al. (1984). After filtration, the pigment was extracted in 90% acetone for 24 h, and kept refrigerated in the dark. After extraction, the fluorescence was measured by spectrofluorometry.
Samples for nutrient analyses were collected at the beginning of each experiment and at alternate days along the duration of the experiments. Samples for determination of nitrate + nitrite, silicate, and phosphate concentrations were kept frozen until analyzed using the standard methods (Hansen and Koroleff 1999) in a Bran Luebbe AA3 AutoAnalyzer. Ammonia concentrations were determined onboard, using a Shimadzu spectrofluorimeter (ICEPOS 2 and ATOS II), following the method described by Kérouel and Aminot (1997), or a SKALAR segmented flow autoanalyzer (LOHAFEX), following the standard procedures. The removal rates of nutrients by the phytoplankton community were estimated as the inverse of the slope obtained from the changes measured in nutrient concentrations with time.
During the ICEPOS 2 experiment, the community composition was determined using epifluorescence microscopy. We sampled 100 mL of each replicate and treatment that were preserved with glutaraldehyde (1% final concentration), filtered onto 0.6 µm Nuclepore filters and kept frozen (−80 °C) until ashore examination in a Zeiss epifluorescence microscope. Phytoplankton cells were grouped into major taxonomic groups (diatoms or flagellates). During the ATOS II experiment, a FlowCAM (FluidImaging Technologies) was used for counting the largest cells on the basis of cell size and fluorescence. The FlowCAM was equipped with a 300 μm-wide flow cell and a camera with a 4× magnification objective, chosen according to the size of the predominant species found in the samples. In subpolar experiments, during LOHAFEX cruise, 100 mL for cell community characterization were preserved by adding approximately 1 mL of Lugol per 250 mL of sample volume, and kept in cold (5 °C, approximately). Prior to microscopic analysis, samples were concentrated following the Utermöhl method in sedimentation chambers for 24 h. Cells were counted in a transmitted-light inverted microscope (Zeiss Axiovert 200) at 20× magnification.
We estimated mean cell volumes of phytoplankton by measuring cell sizes by transmitted-light microscopy (Zeiss inverted microscope) and approximating mathematically to the closest geometric figure. The total biovolume for each group was calculated as the product of cell density and its mean cell volume.
The net growth rates (µ) of the different populations along the experiment were calculated from the slope of changes of the natural logarithm (Ln) of cell abundance N, or the chlorophyll a concentration, over time (t, in days), considering all of the data obtained during the experimental time, by fitting the linear regression equation:
We used the JMP® software for data analysis. The differences in chlorophyll a concentrations and net growth rates between control and excretion treatments and between the different experiments were analyzed using the Student’s t test.
Results
The four communities tested differed in their initial characteristics. Chlorophyll a, ammonia, and silicate concentrations measured at the beginning of each experiment were higher in the communities south of the Antarctic Convergence than in those sampled at lower latitudes (Table 1). Initial nitrate concentrations were lower in our polar experiments, while initial phosphate concentrations were similar amongst experiments (Table 1).
The phytoplankton communities observed during both subpolar experiments (LOHAFEX 1 and 2) were dominated by diatoms, including the genera Thalassiosira, Corethron, Navicula, Cylindrotheca, and Nitzschia, and also by different groups of flagellates (mainly dinoflagellates and Phaeocystis). Cell counts from ATOS II experiment had to be discarded after a malfunction of the cell counting device. During ICEPOS 2, south of the Antarctic Convergence, taxonomic differentiation was low due to the use of epifluorescence microscopy, but we could observe the presence of both pennate and centric diatoms and flagellates, with diatoms dominating the phytoplankton community in terms of abundance and biomass.
The extent of increase in nutrient concentrations with the addition of zooplankton excretion products varied across experiments (Table 2). All communities took up ammonia, with higher net ammonia removal in treatments receiving excretion products when compared to those of control communities (p < 0.01, except for ATOS II). The extent of these increases was related to the correspondent enrichment of ammonia (Table 2; Fig. 2). Communities south of the Antarctic Convergence did not take up both nitrite and nitrate significantly, whereas those north of the polar front showed a higher consumption of these compounds. Nonetheless, no significant increase in net nitrite and nitrate removal rate with the addition of excretion products was observed for any of the communities tested (Table 2; Fig. 2). Phosphate and silicate removal rates were low for the communities south of the Antarctic Convergence and did not increase significantly with the addition of excretion products, but removal rates were enhanced in the communities north of the Antarctic Convergence receiving excretion products, most of all during LOHAFEX exp. 1 (p < 0.01, Table 2; Fig. 2).
Chlorophyll a concentrations increased significantly with time (Student’s t test, p < 0.05), except for the control treatment in ICEPOS 2 (Fig. 3). Net growth rates estimated from chlorophyll a measurements tended to be higher in the communities receiving excretion products in all experiments, with significant differences between treatments in the ICEPOS 2 and LOHAFEX 2 experiments (Student’s t test, p < 0.05, Fig. 4). The results found in the ATOS II experiment were less conclusive than those in the other experiments, likely due to its shorter duration (6 days compared to the 9, 10, and 13 days of incubation during the ICEPOS 2 and both LOHAFEX experiments).
Nonetheless, in all experiments, the net growth rates estimated from chlorophyll a concentration during the first days of incubation are of a similar magnitude, and also showed increased net growth under the excretion treatments.
A wide range of responses to excretion additions was found amongst the different phytoplankton groups. In both subpolar experiments, the net growth of pennate diatoms such as Navicula sp. or Nitzschia sp. in terms of abundance was enhanced at the excretion treatments, and the net growth of Phaeocystis sp. decreased when excretion products were added, although these changes were not significant (data not shown). These differential responses between phytoplankton groups are also reflected in the trends in biovolume estimated for each group in both subpolar experiments (Fig. 5). In the ICEPOS 2 experiments (polar waters), pennate diatoms also increased with the addition of excretion products, although differences with controls were not significant (data not shown).
Discussion
Zooplankton communities are important contributors to the nitrogen (mainly as ammonia) and phosphorus required by phytoplankton for primary production (Alcaraz et al. 2010), playing a key role in recycling nutrients (Vanni 2002), and affecting phytoplankton quantitatively and qualitatively by modifying the pool of available nutrients (Sterner 1986). In fact, there have been observed different temporal and geographic patterns of ammonium distribution related to the complex behavior and physiology of zooplankton influencing their grazing and excretion (Priddle et al. 1997).
In marine systems, the impact of remineralization by microzooplankton on photoautotroph production has been recognized (Burkill et al. 1987; Berman 1991), but during our experiments, we focused on the effect of meso- and macrozooplankton, also clearly important in the overall supply of reduced nitrogen to phytoplankton.
In the Southern Ocean, krill plays a major role as a nutrient recycler, stimulating phytoplankton growth and conditioning the ecosystem to support a high productivity (Tovar-Sanchez et al. 2007; Smetacek 2008). The actual mechanism by which krill is implicated in NH4 + regeneration may not be direct or obvious (Whitehouse et al. 2011), and it has been suggested that krill excretion may enhance bacterial activity (Goeyens et al. 1991; Aristegui et al. 2014), fuelling phytoplankton growth, and thus stimulating periods of high productivity in the Southern Ocean (Smetacek 2008; Aristegui et al. 2014). However, the direct excretion of dissolved ammonium can also be the primary boost to mixed layer NH4 concentrations (Whitehouse et al. 2011; Lehette et al. 2012). High concentrations of krill may supply sufficient local excesses of nutrients to trigger enhanced uptake and phytoplankton turnover (Whitehouse et al. 2011; Lehette et al. 2012).
As observed in our results, the net growth rates of phytoplankton communities estimated as the variation in Chl a concentrations were in general boosted under the excretion treatments, reflecting the stimulating effects of the nutrients excreted by krill, amphipods, and copepods on the net growth of phytoplankton communities. This response has also been observed in previous experiments, with a positive response in phytoplanktonic and bacterial communities (Agustí et al. 2009; Arístegui et al. 2014).
During our subpolar experiments, initial nitrate concentrations were higher than in our Antarctic experiments, so the consumption rates of this nutrient remained higher during the incubation time. During LOHAFEX experiments, excretion additions lead to an increase in ammonia removal rates and a parallel decrease in nitrate removal rates. These opposite trends in ammonia and nitrate removal are not that clear in our Antarctic experiments, as the initial concentrations of ammonia were slightly higher.
Ammonia ambient concentrations are characteristically lower than those of nitrate (Glibert et al. 1982). According to this, and considering that nitrate uptake rates in Antarctic phytoplankton are typically low (Dugdale and Wilkerson 1991) and that the uptake of ammonia is energetically more efficient (Dortch 1990), we found enhanced removal rates of ammonia in the excretion treatments in all experiments. This indicates that ammonia recycling is a major source of nitrogen to phytoplankton (Glibert et al. 1982), particularly so in the Antarctic where nitrate uptake rates are low and ammonium is taken up rapidly (Agustí et al. 2009). The enhanced ammonium uptake under increased ambient ammonium concentrations reflects the preference of phytoplankton for ammonium over nitrate (Olson 1980; Dortch 1990). In fact, previous studies performed in the Scotia Sea observed that ammonium uptake was more than two-thirds of the total inorganic nitrogen assimilated by phytoplankton (Rönner et al. 1983; Koike et al. 1986).
Despite the increase observed in overall phytoplankton biomass (as Chl a, Fig. 4) under excretion addition, our study of the effects of the addition of excretion products on the different phytoplankton groups present in these communities showed a broad range of responses across groups, as the differences observed in Fig. 5. During the iron fertilization experiment that also took place during the LOHAFEX cruise (Smetacek and Naqvi 2010), the initial phytoplankton community was dominated by flagellates, including solitary cells of Phaeocystis sp. After iron addition, a rapid diatom growth was observed that declined within 2–3 weeks after fertilization. This decline could have been due to the depletion of silicic acid to limiting concentrations and/or heavy copepod grazing pressure (Smetacek and Naqvi 2010). During our LOHAFEX experiments, the excretion addition helped to decrease silicon limitation and favored diatom growth.
There has been observed a considerable variation between species in their use of NH4 or nitrate as an N source, influenced by their availability (Seeyave et al. 2009). However, at present, no apparent pattern in the preference for an N source between species can be defined (Dortch 1990), finding different preferences even between similar species (Van Ruth et al. 2012). These different interspecific responses are expected, considering their diverse life-history characteristics, especially in regard to their nutrient requirements (Kilham and Hecky 1988). For instance, several experiments performed in Antarctic waters have described a preference of small phytoplankton for ammonium, compared to large phytoplankton which tend to rely more on nitrate as an N source (Koike et al. 1986; Owens et al. 1991).
It has been suggested that diatoms grow faster and have a higher affinity for nutrients than other phytoplankton taxa under nutrient-replete conditions (Parsons et al. 1978). Sterner (1986) found that nutrient regeneration by Daphnia sp. increased total phytoplankton growth rate as well as that of several taxa; moreover, the taxon responding most positively to nutrient recycling was a group of pennate diatoms. This agrees with the results presented here, where pennate diatoms showed increased net growth rates in the treatments receiving excretion products. This enhanced growth of diatoms would also be reflected in the increased silicate consumption measured in both subpolar experiments, where the initial silicate concentrations were one–two orders of magnitude lower than those in the experiments conducted poleward of the Antarctic Convergence.
Ecologists recognize the importance of consumers in regulating ecosystems processes such as nutrient cycling (Sterner 1986; Elser and Urabe 1999; Lehette et al. 2012), where zooplankton is identified as an important source of recycled nutrients for phytoplankton. The temporal and spatial variability of marine organisms as krill is typically assumed to be ultimately controlled by environmental variability, with large changes in krill biomass observed between regions and years (e.g., Atkinson et al. 2004; Siegel 2005; Alcaraz et al. 2014). The distribution of krill varies horizontally and also along the year; west of the Antarctic Peninsula, most winter observations are characterized by the absence of krill, with low biomass aggregations occurring deeper in the water column (Lascara et al. 1999). Krill also shows ontogenetic and diel vertical migration, feeding in the phytoplankton-rich shallow waters during darkness and avoiding predation by residing deeper during daylight (Demer and Hewitt 1995).
Several studies have described a decline in krill standing sock associated with reduced ice cover during the last decades (Loeb et al. 1997; Atkinson et al. 2004). In fact, despite the high krill concentrations previously reported close to the Antarctic Peninsula and in the Scotia Sea, krill populations have declined twofold since the mid-1970s due to profound changes along this area (Atkinson et al. 2004). This key species for the trophic network of the Southern Ocean has also been influenced by the emerging Antarctic krill fishery and significant ecological changes during the second half of the twentieth century (Murphy et al. 2007; Santana et al. 2013).
Besides this krill decline, differences in the stoichiometry of excretion products are potentially important in determining the relative degree of nutrient limitation and thus algal species composition (Vanni 2002). It has been described that environmental changes can affect the ratios at which the different nutrients are recycled by krill, in example, predicting increased NH4 +, DOC, and PO4 + release rates in a high CO2 scenario (Saba et al. 2012).
In parallel, the decline of krill populations seems to be leading to a substitution by salps as the main components of the zooplankton community (Smetacek and Nicol 2005). Although the excretion of salps also contributes greatly to nutrient recycling and supply to phytoplankton, the stoichiometric ratios of the excretion products differ greatly between salps and krill (Alcaraz et al. 2014). The ratios at which nutrients are recycled can exert a strong selective effect in natural communities of phytoplankton (Hecky and Kilham 1988), leading to strong indirect effects in natural communities (Sterner 1986), suggesting that the shift from krill to salps may also select for phytoplankton communities with stoichiometric ratios more similar to those of the salp excretion products.
It has been observed that the world ocean has undergone a warming process since the mid-20th century (Levitus et al. 2000), including remote areas such as the surroundings of the Antarctic Peninsula and near-coastal Antarctica (Christensen et al. 2007).
In a future scenario under increased temperatures, with a decreased ice cover and an increase in thermal stratification, nutrient input from deeper water masses may be diminished, and the role of zooplankton as nutrient recyclers may become more important as ammonia sources for phytoplankton. This way, excretion and migration may impose a temporal pattern on ammonium concentration and distribution, being an effective means of transporting nitrogen through the mixed layer (Priddle et al. 1997).
If the changes in the rates of zooplankton excretion persist, the stoichiometry and the quality of the dissolved pool of nutrients available for phytoplankton may be modified. As observed here, this would imply a decrease in total phytoplanktonic biomass, as zooplankton excretion usually seeds the conditions for subsequent phytoplankton blooms (Agustí et al. 2009). Changes in the phytoplankton community composition may also be induced, as the form in which nitrogen is available for phytoplankton can have an influence on the productivity of different size classes and ultimately affect the sinking rates of the community (Stolte et al. 1994).
This will contribute to induce the abrupt, non-linear changes expected on the community structure of plankton ecosystems (Alcaraz et al. 2010). Thus, the decline in krill standing stock reported in the Southern Ocean may have deeper effects in the Southern Ocean phytoplankton than believed (Ruiz-Halpern et al. 2011) if these populations are deprived of the stimulating effects of krill excretion, even altering the carbon export, sustained on recycled nitrogen (Priddle et al. 1995).
References
Agustí S, Duarte CM (2000) Experimental induction of a large phytoplankton bloom in Antarctic coastal waters. Mar Ecol Prog Ser 206:73–85
Agustí S, Duarte CM, Llabrés M, Agawin NS, Kennedy H (2009) Response of coastal Antarctic phytoplankton to solar radiation and ammonium manipulation: An in situ mesocosm experiment. J Geophys Res Biogeosci 114:G01009. doi:10.1029/2008JG000753
Alcaraz M, Saiz E, Fernandez JA, Trepat I, Figueiras F, Calbet A, Bautista B (1998) Antarctic zooplankton metabolism: carbon requirements and ammonium excretion of salps and crustacean zooplankton in the vicinity of the Bransfield Strait during January 1994. J Mar Syst 17:347–359
Alcaraz M, Almeda R, Calbet A, Saiz E, Duarte CM, Lasternas S, Agustí S, Santiago R, Movilla J, Alonso A (2010) The role of arctic zooplankton in biogeochemical cycles: respiration and excretion of ammonia and phosphate during summer. Polar Biol 33:1719–1731
Alcaraz M, Almeda R, Duarte CM, Horstkotte B, Lasternas S, Agustí S (2014) Changes in the C, N, and P cycles by the predicted salps-krill shift in the Southern Ocean. Front Mar Sci 1:45
Arístegui J, Duarte CM, Reche I, Gómez-Pinchetti JL (2014) Krill excretion boosts microbial activity in the Southern Ocean. PloS One 9:e89391
Arrigo KR, Robinson DH, Worthen DL, Dunbar RB, DiTullio GR, VanWoert M, Lizotte MP (1999) Phytoplankton community structure and the drawdown of nutrients and CO2 in the Southern Ocean. Science 283:365–367
Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Berman T (1991) Protozoans as agents in planktonic nutrient cycling. In: Reid PC, Turley CM, Burkill PH (ed) Protozoa and their role in marine processes. Springer, Berlin, pp 417–429
Biggs DC (1982) Zooplankton excretion and NH4 + cycling in near-surface waters of the Southern Ocean. I. Ross Sea, Austral Summer 1977–1978. Polar Biol 1:55–67
Brandini FP, Baumann MEM (1997) The potential role of melted “brown ice” as sources of chelators and ammonia to the surface waters of the Weddell Sea, Antarctica. Proc NIPR Symp. Polar Biol 10:1–13
Burkill JH, Mantoura RFC, Llewellyn CA, Owens NJP (1987) Microzooplankton grazing and selectivity of phytoplankton in coastal waters. Mar Biol 93:581–590
Christensen JH, Hewitson B, Busuioc A et al (2007) Regional climate projections. In: Solomon S, Qin D, Manning M, Chen Z et al (eds) IPCC climate change 2007: the physical science basis. Cambridge Univ. Press, Cambridge, pp 849–940
Clarke A, Leakey RJ (1996) The seasonal cycle of phytoplankton, macronutrients, and the microbial community in a nearshore Antarctic marine ecosystem. Limnol Oceanogr 41:1281–1294
Corner EDS, Davies AG (1971) Plankton as a factor in the nitrogen and phosphorus cycles in the sea. Adv Mar Biol 9:101–204
Demer DA, Hewitt RP (1995) Bias in acoustic biomass estimates of Euphausia superba due to diel vertical migration. Deep Sea Res Part I Oceanogr Res Pap 42:455–475
de Santana CN, Rozenfeld AF, Marquet PA, Duarte CM (2013) Topological properties of Polar food webs. Mar Ecol Progr Ser 474:15–26
Dortch Q (1990) The interaction between ammonium and nitrate uptake in phytoplankton. Mar Ecol Prog Ser 61:183–201
Dugdale RC, Wilkerson FP (1991) Low specific nitrate uptake rate: A common feature of high-nutrient, low-chlorophyll marine ecosystems. Limnol Oceanogr 36:1678–1688
El-Sayed S (1984) Productivity of the Antarctic waters—a reappraisal. In: Holm-Hansen O, Bolis L, Gilles R (eds) Marine phytoplankton and productivity. Springer, Berlin, pp 11–50
Elser JJ, Urabe J (1999) The stoichiometry of consumer-driven nutrient recycling: theory, observations, and consequences. Ecology 80:735–751
Fiala M, Semeneh M, Oriol L (1998) Size-fractionated phytoplankton biomass and species composition in the Indian sector of the Southern Ocean during austral summer. J Mar Syst 17:179–194
Garibotti IA, Vernet M, Smith RC, Ferrario ME (2005) Interannual variability in the distribution of the phytoplankton standing stock across the seasonal sea-ice zone west of the Antarctic Peninsula. J Plankton Res 27:825–843
Glibert PM, Douglas CB, McCarthy JJ (1982) Utilization of ammonium and nitrate during austral summer in the Scotia Sea. Deep-Sea Res 29:837–850
Goeyens L, Tréguer P, Lancelot C, Mathot S, Becquevort S, Morvan J, Dehairs F, Baeyens W (1991) Ammonium regeneration in the Scotia-Weddell Confluence area during spring 1988. Mar Ecol Prog Ser 78:241–252
Hansen K, Koroleff FF (1999) Determination of nutrients. In: Grasshoff K, Kremling K, Ehrhardt M (eds) Methods of seawater analysis. Wiley, Germany, pp 159–228
Hecky RE, Kilham P (1988) Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol Oceanogr 33:796–822
Kérouel R, Aminot A (1997) Fluorometric determination of ammonia in sea and estuarine waters by direct segmented flow analysis. Mar Chem 57:265–275
Kilham P, Hecky RE (1988) Comparative ecology of marine and freshwater phytoplankton. Limnol Oceanogr 33:776–795
Koike I, Holm-Hansen O, Biggs DC (1986) Inorganic nitrogen metabolism by Antarctic phytoplankton with special reference to ammonium cycling. Mar Ecol Prog Ser 30:105–116
Kopczyńska EE, Savoye N, Dehairs F, Cardinal D, Elskens M (2007) Spring phytoplankton assemblages in the Southern Ocean between Australia and Antarctica. Polar Biol 31:77–88
Lascara CM, Hofmann EE, Ross RM, Quetin LB (1999) Seasonal variability in the distribution of Antarctic krill, Euphausia superba, west of the Antarctic Peninsula. Deep Sea Res Part I Oceanogr Res Pap 46:951–984
Lehette P, Tovar-Sánchez A, Duarte CM, Hernández-León S (2012) Krill excretion and its effect on primary production. Mar Ecol Prog Ser 459:29–38
Levitus S, Antonov JI, Boyer TP, Stephens C (2000) Warming of the world ocean. Science 287:2225–2229
Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic foodweb. Nature 387:897–900
Mackey AP, Atkinson A, Hill SL, Ward P, Cunningham NJ, Johnston NM, Murphy EJ (2012) Antarctic macrozooplankton of the southwest Atlantic sector and Bellingshausen Sea: Baseline historical distributions (Discovery Investigations, 1928–1935) related to temperature and food, with projections for subsequent ocean warming. Deep Sea Res Part II Top Stud Oceanogr 59:130–146
Marrari M, Daly KL, Hu C (2008) Spatial and temporal variability of SeaWiFS chlorophyll a distributions west of the Antarctic Peninsula: Implications for krill production. Deep Sea Res Part II Top Stud Oceanogr 55:377–392
Murphy EJ, Watkins JL, Trathan PN et al (2007) Spatial and temporal operation of the Scotia Sea ecosystem: a review of large-scale links in a krill centred food web. Philos Trans R Soc Lond B Biol Sci 362:113–148
Olson RJ (1980) Nitrate and ammonium uptake in Antarctic waters. Limnol Oceanogr 25:1064–1074
Owens NJP, Priddle J, Whitehouse MJ (1991) Variations in phytoplanktonic nitrogen assimilation around South Georgia and in the Bransfield Strait (Southern Ocean). Mar Chem 35:287–304
Parsons TR, Harrison PJ, Waters R (1978) An experimental simulation of changes in diatom and flagellate blooms. J Exp Mar Biol Ecol 32:285–294
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford
Priddle J, Leakey R, Symon C et al (1995) Nutrient cycling by Antarctic marine microbial plankton. Mar Ecol Prog Ser 116:181–198
Priddle J, Whitehouse MJ, Atkinson A, Brierley AS, Murphy EJ (1997) Diurnal changes in near-surface ammonium concentration—interplay between zooplankton and phytoplankton. J Plankton Res 19:1305–1330
Probyn TA, Painting SJ (1985) Nitrogen uptake by size-fractionated phytoplankton populations in Antarctic surface waters. Limnol Oceanogr 30:1327–1332
Rönner U, Sörensson F, Holm-Hansen O (1983) Nitrogen assimilation by phytoplankton in the Scotia Sea. Polar Biol 2:137–147
Ruiz-Halpern S, Duarte CM, Tovar-Sánchez A, Pastor M, Horstkotte B, Lasternas S, Agusti S (2011) Antarctic krill as a source of dissolved organic carbon to the Antarctic ecosystem. Limnol Oceanogr 56:521–528
Saba GK, Schofield O, Torres JJ, Ombres EH, Steinberg DK (2012) Increased feeding and nutrient excretion of adult Antarctic krill, Euphausia superba, exposed to enhanced carbon dioxide (CO2). PloS One 7:e52224
Sakshaug E, Holm-Hansen O (1984) Factors governing pelagic production in polar oceans. In: Holm-Hansen O, Bolis L, Gilles R (eds) Marine phytoplankton and productivity. Springer, Berlin, pp 11–50
Sañudo-Wilhelmy SA, Olsen KA, Scelfo JM, Foster TD, Flegal AR (2002) Trace metal distributions off the Antarctic Peninsula in the Weddell Sea. Mar Chem 77:157–170
Sarthou G, Bucciarelli E, Chever F, Hansard SP, González-Dávila M, Santana-Casiano JM, Planchon F, Speich S (2011) Labile Fe(II) concentrations in the Atlantic sector of the Southern Ocean along a transect from the subtropical domain to the Weddell Sea Gyre. Biogeosciences 8:2461–2479
Seeyave S, Probyn TA, Pitcher GC, Lucas MI, Purdie DA (2009) Nitrogen nutrition in assemblages dominated by Pseudo-nitzschia spp., Alexandrium catenella and Dinophysis acuminata off the west coast of South Africa. Mar Ecol Prog Ser 379:91–107
Siegel V (2005) Distribution and population dynamics of Euphausia superba: summary of recent findings. Polar Biol 29:1–22
Smetacek V (2008) Are declining Antarctic krill stocks as result of global warming or of the decimation of he whales? In: Duarte CM (ed) Impacts of Global Warming on Polar Ecosystems. Fundación BBVA, pp 45–83
Smetacek V, Naqvi SWA (2010) The expedition of the research vessel” Polarstern” to the Antarctic in 2009 (ANT-XXV/3-LOHAFEX). Alfred Wegener Institute for Polar and Marine Research, Bremerhaven
Smetacek V, Nicol S (2005) Polar ocean ecosystems in a changing world. Nature 437:362–368
Steinberg DK, Ruck KE, Gleiber MR et al (2015) Long-term (1993–2013) changes in macrozooplankton off the Western Antarctic Peninsula. Deep Sea Res Part I Oceanogr Res Pap 101:54–70
Sterner RW (1986) Herbivores’ direct and indirect effects on algal populations. Science 231:605–607
Stolte W, McCollin T, Noordeloos AA, Riegman R (1994) Effect of nitrogen source on the size distribution within marine phytoplankton populations. J Exp Mar Biol Ecol 184:83–97
Tatur A, Myrcha A (1983) Changes in chemical composition of waters running off from the penguin rookeries in the Admiralty Bay region (King George Island, South Shetland Islands, Antarctica). Pol Polar Res 4:113–126
Tovar-Sánchez A, Duarte CM, Hernández-León S, Sañudo-Wilhelmy SA (2007) Krill as a central node for iron cycling in the Southern Ocean. Geophys Res Lett 34:L11601
Van Ruth PD, Qin JG, Branford AJ (2012) Size dependent competition in centric diatoms as a function of nitrogen and silicon availability. Open J Marine Sci 2:33–42
Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Evol Syst 33:341–370
Whitehouse MJ, Atkinson A, Rees AP (2011) Close coupling between ammonium uptake by phytoplankton and excretion by Antarctic krill, Euphausia superba. Deep Sea Res Part I Oceanogr Res Pap 58:725–732
Acknowledgements
This is a contribution to the projects ATOS (Aportes Atmosféricos de Carbono Orgánico y Contaminantes al Océano Polar) and ICEPOS (REN2002-04165-C03-02⁄ANT) funded by the Spanish Ministry of Education and Science, and to the LOHAFEX project, funded by the Max Planck Society. We thank S. W. A. Naqvi, chief scientist of the LOHAFEX project, for his leadership, Victor Smetacek, Regino Martínez for experiment setup and sampling, and Maria Grazia Mazzocchi (Stazione Zoologica Anton Dohrn, Napoli, Italy) for help with zooplankton information. We also thank the crew of the RV Polarstern and BIO Hespérides for their help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Coello-Camba, A., Llabrés, M., Duarte, C.M. et al. Zooplankton excretion metabolites stimulate Southern Ocean phytoplankton growth. Polar Biol 40, 2035–2045 (2017). https://doi.org/10.1007/s00300-017-2123-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2123-2