Abstract
Key message
Freezing tolerance in taft plants relied more upon an ABA-independent- than an ABA-dependent antifreeze signaling pathway.
Abstract
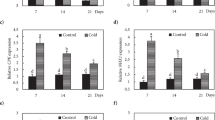
Two wheat (Triticum aestivum) near isogenic lines (NIL) named tafs (freezing sensitivity) and taft (freezing tolerance) were isolated in the laboratory and their various cytological and physiological characteristics under freezing conditions were studied. Proplastid, cell membrane, and mitochondrial ultrastructure were less damaged by freezing treatment in taft than tafs plants. Chlorophyll, ATP, and thylakoid membrane protein contents were significantly higher, but malondialdehyde content was significantly lower in taft than tafs plants under freezing condition. Antioxidant capacity, as indicated by reactive oxygen species accumulation and antioxidant enzyme activity, and the relative gene expression were significantly greater in taft than tafs plants. Soluble sugars and abscisic acid (ABA) contents were significantly higher in taft plants than in tafs plants under both normal and freezing conditions. The upregulated expression levels of certain freezing tolerance-related genes were greater in taft than tafs plants under freezing treatment. The addition of sodium tungstate, an ABA synthesis inhibitor, led to only partial freezing tolerance inhibition in taft plants and the down-regulated expression of some ABA-dependent genes. Thus, both ABA-dependent and ABA-independent signaling pathways are involved in the freezing tolerance of taft plants. At the same time, freezing tolerance in taft plants relied more upon an ABA-independent- than an ABA-dependent antifreeze signaling pathway.











Similar content being viewed by others
Abbreviations
- NIL:
-
Near isogenic lines
- MDA:
-
Malondialdehyde
- NBT:
-
Nitroblue tetrazolium
- DAB:
-
Diaminobenzidine
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- POD:
-
Guaiacol peroxidase
- Glu:
-
Glucose
- Fru:
-
Fructose
- Suc:
-
Sucrose
- ABA:
-
Abscisic acid
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
References
Akter K, Kato M, Sato Y, Kaneko Y, Takezawa D (2014) Abscisic acid-induced rearrangement of intracellular structures associated with freezing and desiccation stress tolerance in the liverwort Marchantia polymorpha. J Plant Physiol 171:1334–1343
Carpenter JF, Crowe JH (1988) The mechanism of cryoprotection of protein solutes. Cryobiology 25:244–255
Chen G, Liu S, Zhang C, Lu C (2004) Effects of drought on photosynthetic characteristics of flag leaves of a newly-developed super-high-yield rice hybrid. Photosynthetica 42:573–578
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
Danquah A, de Zelicourt A, Colcombet J, Hirt H (2014) The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv 32:40–52
Degenhardt B, Gimmler H, Hose E, Hartung W (2000) Effect of alkaline and saline substrates on ABA content, distribution and transport in plant roots. Plant Soil 225:83–94
Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A (2014) Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J Exp Bot 65:1259–1270
Dhillon T, Pearce ST, Stockinger EJ, Distelfeld A, Li CX, Knox AK (2010) Regulation of freezing tolerance and flowering in temperate cereals: the VRN-1 connection. Plant Physiol 153:1846–1858
Ding YL, Li H, Zhang XY, Xie Q, Yang SH (2015) OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev Cell 32:278–289
Fowler S, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14:1675–1690
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Foyer CH, Vanacker H, Gomez LD, Harbinson J (2002) Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: review. Plant Physiol Biochem 40:659–668
Frederiks TM, Christopher JT, Harvey GL, Sutherland MW (2012) Current and emerging screening methods to identify post-head emergence freezing adaptation in wheat and barley. J Exp Bot 63:5405–5416
Gill BS, Appels R, Botha-Oberholster AM, Buell CR (2004) A workshop report on wheat genome sequencing: international genome research on wheat consortium. Genetics 168:1087–1096
Guajardo E, Correa JA, Contreras-Porcia L (2016) Role of abscisic acid (ABA) in activating antioxidant tolerance responses to desiccation stress in intertidal seaweed species. Planta 243:767–781
Guy CL (1990) Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Biol 41:187–223
Hui Z, Tian FX, Wang GK, Wang W (2012) The antioxidative defense system is involved in the delayed senescence in a wheat mutant tasg1. Plant Cell Rep 31:1073–1084
Klotke J, Kopka J, Gatzke N, Heyer AG (2004) Impact of soluble sugar concentrations on the acquisition of freezing tolerance in accessions of Arabidopsis thaliana with contrasting cold adaptation evidence for a role of raffinose in cold acclimation. Plant Cell Environ 27:1395–1404
Kobayashi F, Takumi S, Nakamura C (2008) Increased freezing tolerance in an ABA-hypersensitive mutant of common wheat. J Plant Physiol 165:224–232
Le Martret B, Poage M, Shiel K, Nugent GD, Dix PJ (2011) Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol J 9:661–673
Li YJ, Fang Y, Fu YR, Huang JG, Chang-Ai Wu CA, Zheng CC (2013) NFYA1 is involved in regulation of postgermination growth arrest under salt stress in Arabidopsis. PLoS One 8(e6):1289
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Maruyama K, Sakuma Y, Kasuga M, Ito Y, Seki M, Goda H, Shimada Y (2004) Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J 38:982–993
Mayer BF, Benalia MAA, Demonea J, Bertrandb A (2015) Cold acclimation induces distinctive changes in the chromatin state and transcript levels of COR genes in Cannabis sativa varieties with contrasting cold acclimation capacities. Physiol Plant 155:281–295
Nagao M, Minami A, Arakawa K, Fujikawa S, Takezawa D (2005) Rapid degradation of starch in chloroplasts and concomitant accumulation of soluble sugars associated with ABA-induced freezing tolerance in the moss Physcomitrella patens. J Plant Physiol 162:169–180
Novák A, Boldizsár Á, Ádám É, Kozma-Bognár L, Majláth I (2016) Light-quality and temperature-dependent CBF14 gene expression modulates freezing tolerance in cereals. J Exp Bot 67:1285–1295
Parida AK, Das AB, Mittra B (2003) Effects of NaCl stress on the structure, pigment complex composition, and photosynthetic activity of mangrove Bruguiera parviflora chloroplasts. Photosynthetica 41:191–200
Parvanova D, Ivanov S, Konstantinova T, Karanov E, Atanassov A (2004) Transgenic tobacco plants accumulating osmolytes show reduced oxidative damage under freezing stress. Plant Physiol Biochem 42:57–63
Pearce RS (1999) Molecular analysis of acclimation to cold. Plant Growth Regul 29:47–76
Ploschuk EL, Bado LA, Salinas M, Wassner DF, Windauer LB (2014) Photosynthesis and fluorescence responses of Jatropha curcas to chilling and freezing stress during early vegetative stages. Environ Exp Bot 102:18–26
Poppek D, Grune T (2006) Proteasomal defense of oxidative protein modifications. Antioxid Redox Signal 8:173–184
Quan RD, Shang M, Zhang H, Zhao YX (2004) Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci 166:141–149
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Ruelland E, Vaultier MN, Zachowski A, Hurry V (2009) Cold signalling and cold acclimation in plants. Adv Bot Res 49:35–150
Sairam PK, Srivastava GC (2002) Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162:897–904
Sasaki K, Christov NK, Tsuda S, Imai R (2014) Identification of a novel LEA protein involved in freezing tolerance in wheat. Plant Cell Physiol 55:136–147
Sehrawat A, Gupta R, Deswal R (2013) Nitric oxide-cold stress signalling cross-talk, evolution of a novel regulatory mechanism. Proteomics 13:1816–1835
Seo PJ, Kim MJ, Park JY, Kim SY (2010) Cold activation of a plasma membrane-tethered NAC transcription factor induces a pathogen resistance response in Arabidopsis. Plant J 61:661–671
Tarkowski LP, Van den Ende W (2015) Cold tolerance triggered by soluble sugars: a multifaceted countermeasure. Front Plant Sci 6:203
Thalhammer A, Bryant G, Sulpice R, Hincha DK (2014) Disordered cold regulated15 proteins protect chloroplast membranes during freezing through binding and folding, but do not stabilize chloroplast enzymes in vivo. Plant Physiol 166:190–201
Theocharis A, Clément C, Barka EA (2012) Physiological and molecular changes in plants grown at low temperatures. Planta 235:1091–1105
Tian FX, Gong JF, Zhang J, Wang W (2013) Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. J Exp Bot 64:1509–1520
Tsuda K, Tsvetanov S, Takumi S, Mori N, Atanas Atanassov A (2000) New members of a cold-responsive group-3 Lea/Rab-related Cor gene family from common wheat (Triticum aestivum L.). Genes Genet Syst 75:179–188
Uno Y, Furihata T, Abe H, Yoshida R (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. PNAS 97:11632–11637
Velicka R, Rimkeviciene M, Novickiene L, Anisimoviene N, Brazauskiene I (2005) Improvement of oil rape hardening and frost tolerance. Russ J Plant Physiol 52:473–480
Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF (2005) Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J 41:195–211
Waalen WM, Stavang JA, Olsen JE, Rognli OA (2014) The relationship between vernalization saturation and the maintenance of freezing tolerance in winter rapeseed. Environ Exp Bot 106:164–173
Wang WQ, Hao QQ, Tian FX, Li QX, Wang W (2016) The stay-green phenotype of wheat mutant tasg1 is associated with altered cytokinin metabolism. Plant Cell Rep 35:585–599
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cisacting regulatory elements in osmotic- and coldstress-responsive promoters. Trends Plant Sci 10:88–94
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514
Zhang LL, Zhao MG, Tian QY, Zhang WH (2011) Comparative studies on tolerance of Medicago truncatula and Medicago falcata to freezing. Planta 234:445–457
Zheng CF, Jiang D, Liu FL, Dai TB (2009a) Effects of salt and waterlogging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Sci 176:575–582
Zheng YL, Feng YL, Lei YB, Yang CY (2009b) Different photosynthetic responses to night chilling among twelve populations of Jatropha curcas. Photosynthetica 47:559–566
Zhu XL, Qi L, Liu X, Cai SB, Xu HJ (2014) The wheat ethylene response factor transcription factor pathogen-induced erf1 mediates host responses to both the necrotrophic pathogen rhizoctonia cerealis and freezing stresses. Plant Physiol 164:1499–1514
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31370304) and by Funds of Shangdong “Double Tops” Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Communicated by Chun-Hai Dong.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2017_2195_MOESM1_ESM.tif
Fig. S1. Thylakoid polypeptides of tafs and taft under control conditions (25°C) and freezing treatment (–20°C) (a), and relative signal densities of corresponding samples of SDS-PAGE in panels (b). M represents molecular mass marker used for SDS-PAGE. Values are mean ± SD based on three replicates. Error bars indicate standard deviations (TIFF 1795 kb)
Rights and permissions
About this article
Cite this article
Wang, W., Hao, Q., Wang, W. et al. The genetic characteristics in cytology and plant physiology of two wheat (Triticum aestivum) near isogenic lines with different freezing tolerances. Plant Cell Rep 36, 1801–1814 (2017). https://doi.org/10.1007/s00299-017-2195-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2195-z