Abstract
Endothelial dysfunction (ED) is defined as an impairment in the vasodilatory, anti-thrombotic, and anti-inflammatory properties of the cells that make up the lining of blood vessels. ED is considered a key step in the development of atherosclerotic cardiovascular disease. The association between ED and systemic inflammatory diseases is well established. However, the prevalence and clinical significance of ED in psoriatic arthritis (PsA) have been investigated to a lesser extent. This review aims to explore the link between ED and PsA, including ED in macro- and microcirculation, as well as risk factors for its occurrence in PsA and its relationship with atherosclerosis in PsA. Furthermore, the ED in PsA was compared with that of rheumatoid arthritis (RA). Regarding ED in the microcirculation, the coronary flow reserve was found to be significantly reduced in individuals with PsA. The relationship between PsA and macrovascular ED is more pronounced, along with more advanced atherosclerosis detected in patients with PsA. These results are consistent with those obtained in RA studies. On the other hand, arterial stiffness and signs of vascular remodeling were found more frequently in RA than in PsA, with the potential role of efficient anti-TNF treatment in patients with PsA and psoriasis explaining this finding. The impact of ED on cardiovascular diseases and the burden of this risk caused independently by PsA have not yet been precisely established, however, this group of patients requires special attention with regard to cardiovascular events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory musculoskeletal disorder that often accompanies psoriasis (PsO). PsO affects 1–3% of the general population, and up to 30% of these individuals develop PsA [1, 2]. The American College of Rheumatology recognized PsA as a distinct clinical entity in the 1960s. Initially perceived as benign, recent findings indicate that the severity of PsA is comparable to that of rheumatoid arthritis (RA), significantly affecting quality of life and joint functionality. PsA can manifest in various forms, such as axial or peripheral arthritis, enthesitis, dactylitis, or a combination of these [3]. Approximately 90% of patients with PsA exhibit typical dermatological symptoms of PsO, although PsA can arise without skin manifestations [2]. In about 75% of cases, PsA is preceded by PsO, but in 10–15% of cases, the sequence may be reversed. The occurrence of PsA without PsO is also possible and may pose a diagnostic challenge [4]. Both RA and PsA share several features, such as chronic progression, inflammatory symptoms, and an association with increased cardiovascular (CV) morbidity and mortality, including a higher prevalence of traditional cardiovascular risk factors (CVRF) compared to controls [5]. Endothelial dysfunction (ED), a key link between RA, PsA, and CV diseases, is an early indicator of atherosclerosis [6] and plays a significant role in the progression of RA, PsA, and related comorbidities. Studies have highlighted the association of PsA with increased CV morbidity and mortality [7, 8]. Although patients with PsA often have classical CVRF such as diabetes, hypertension, hyperlipidemia, obesity, and smoking [8, 9], there are still other factors to consider in the pathogenesis of atherosclerotic cardiovascular disease (ASCVD) and, consequently, CV mortality. Despite similarities, RA and PsA differ in terms of CV risk burden and CV comorbidity profiles. These differences are also reflected in the results of studies investigating ED and signs of subclinical atherosclerosis and vascular remodeling. The impact of PsA and RA on the endothelium has been the subject of numerous studies. This article synthesizes peer-reviewed literature on the impact of PsA on endothelial function in both macrocirculation and microcirculation, comparing it with RA to provide a comprehensive perspective.
Search strategy
Considering previously published recommendations, our strategy was to search for accessible studies focusing on ED in patients with PsA and patients with RA, published by September 1, 2023. To ensure a comprehensive and thorough data collection, we utilized a range of online databases including MEDLINE, Cochrane and EMBASE. Additionally, we employed Google Scholar as a secondary verification tool to cross-check and augment our search results. The search methodology incorporates specific commands that integrate the phrases "psoriatic arthritis" AND "endothelial dysfunction". For a more advanced search, the methodology combines the name of the disease with specific indicators that evaluate endothelial dysfunction across various levels. These indicators include measure in microcirculation—coronary flow reserve, and in macrocirculation, which encompasses assessments like flow-mediated dilatation, carotid total plaque area, coronary plaque burden, and carotid plaque burden. Additionally, the search extends to evaluating arterial stiffness, employing metrics: the augmentation index, pulse-wave velocity, and carotid intima-media thickness. Furthermore, the bibliographies of the retrieved articles were examined to identify the validated literature.
Given the innovative approach to the topic, the inclusion criteria were intentionally broadened to maximize the scope of the study concerning ED among PsA patients. However, several potential confounding factors were assessed, including comorbidities in PsA patients, medication intake, the absence of a specified inflammatory joint disease, or the simultaneous assessment of several joint diseases in one study group without subgroup analysis. Additionally, significant limitations of the studies were reviewed, such as the failure to distinguish PsA patients from those with psoriasis, treating them as a single group, and the lack of information regarding the patients' current treatment stage (whether the disease is controlled or newly diagnosed, etc.). Comparative indicators for RA were chosen for comparative purposes without special selection criteria, beyond assessing the study's internal validity.
Given the limited availability of specific data, we used the findings on the status of ED in RA as a benchmark for comparative analysis, provided that these findings were not affected by publication bias. This approach was aimed at strengthening the validity of our results [10].
Endothelial dysfunction in PsA
Coronary flow reserve
Coronary flow reserve (CFR) is a highly sensitive marker that can predict the occurrence of severe coronary stenosis and is a diagnostic marker of coronary artery disease (CAD) [5]. The work by Atzeni et al. highlighted a notable reduction in CFR among patients with PsA (2.86 ± 0.70 compared to a normal range of 3.3 ± 0.43; p < 0.01) [11]. This finding is consistent with the related work that demonstrated a significant reduction in CFR in patients with PsA compared to healthy individuals (1.9 ± 0.3 vs 3.6 ± 0.2; p < 0.002) [12]. Retrospective analysis research carried out in patients with PsO among whom there were also individuals with PsA further corroborated these findings. The study identified coronary microvascular dysfunction, defined as CFR < 2.5, and found that it is associated with more severe PsO (OR = 3.1; p = 0.03) and the presence of PsA (OR = 2.9; p = 0.03) [13]. These observations are supported by other researchers [14,15,16]. Furthermore, elevated levels of asymmetric dimethylarginine (ADMA) in patients with PsA have been found to correlate with significantly reduced CFR, indicating ED in central microcirculation, similar to the findings in patients with RA. In a study by Puig et al., the impact of tumor necrosis factor inhibitor (TNFi) therapy on CFR was investigated. In patients with PsO but with no PsA without cardiovascular disease, CFR measurements in the left anterior descending coronary artery showed a significant increase after an average of 6 months of TNFi treatment, compared with baseline values [17]. This improvement in CFR was associated with reduced levels of TNF and high-sensitivity C-reactive protein (CRP), although it did not correspond to changes in the Psoriasis Area and Severity Index (PASI) [17]. This points to a discrepancy between the severity of ED and skin involvement (Table 1).
Flow-mediated dilatation
Flow-mediated dilation (FMD) of the brachial artery is a reliable method for assessing nitric oxide (NO) dependent endothelial function in conduit arteries and early stages of atherosclerosis [6, 18]. Numerous studies have consistently shown that FMD is significantly impaired in patients with PsA compared to healthy individuals. This reduction in FMD has been observed even in patients without traditional CVRF, including in cases of juvenile PsA [19,20,21,22]. These findings support the idea of a higher incidence of macrovascular ED in PsA, mainly in the form of impaired NO release, as suggested by preserved nitroglycerin-mediated dilatation (NMD). Correction of a decrease in FMD [%] after sublingual nitroglycerin administration was observed in two independent studies [20, 23]. These results showed a strong correlation with CRP levels, a known factor in promoting ASCVD in part through the downregulation of endothelial nitric oxide synthase transcription [24]. The other important factor that contributes to the impaired NO release may be elevated levels of ADMA, an endogenous inhibitor of endothelial nitric oxide synthase, which has been found to be increased in ASCVD [25] and in patients with PsA [11]. It is considered a possible marker of subclinical ASCVD, however, its levels were noted to decrease in RA patients after treatment. This finding suggests that ADMA levels may not be elevated in patients with PsA treated with disease-modifying anti-rheumatic drugs (DMARDs) or biologic therapies [11, 19].
Arterial stiffness in PsA
Augmentation index (AIx)
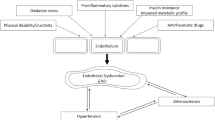
The augmentation index (AIx) is a marker of arterial stiffness. Its increase is one of the first signs of vascular dysfunction. Shang et al. showed that individuals with PsA have higher AIx values as compared to healthy controls [26], however, those results have not been replicated in other studies. In addition, conflicting data have been obtained regarding the association between disease activity and AIx. In research by Angel et al., a mixed group of patients with RA, PsA, and ankylosing spondylitis was evaluated. It was revealed that fluctuations in disease activity, associated with cyclical character of long-term infliximab treatment, were not accompanied by significant changes in arterial stiffness indices, including AIx [28]. However, two studies that evaluated the importance of the treat-to-target approach solely in PsA, showed that achieving a sustained minimal disease activity (sMDA) and sustained low disease activity, as measured by the PsA Disease Activity Score (PASDAS), resulted in reduction of AIx [29, 30]. Therefore, due to inconsistent results, drawing strong conclusions about the relationship between PsA and AIx is challenging (Fig. 1).
A Comparative analysis of endothelial dysfunction (ED) and subclinical atherosclerosis in psoriatic arthritis (PsA) and rheumatoid arthritis (RA). This figure visualizes the outcomes of endothelial dysfunction and subclinical atherosclerosis tests, comparing PsA with RA. Each disease is distinguished by specific colors for easy identification. An upward arrow indicates results higher than those of healthy controls, signifying elevated levels, while a downward arrow indicates reduced levels. A question mark denotes inconclusive outcomes. The test names are clearly labeled within frames for quick reference. CTP carotid total plaque, CFR coronary flow reserve, PWV pulse wave velocity, CIMT carotid intima media thickness, NFC nailfold capillaroscopy, FMD flow-mediated dilatation, Aix Augmentation Index Creation Details: The figure was designed using Microsoft PowerPoint and Canva (https://www.canva.com/pl_pl/) on February 3, 2024. Online access at https://www.canva.com/design/DAF7vdCJ41s/aCvx1TqqhiFH-V0dLY-lVw/view. Certain illustrations within the figure are adapted from Servier Medical Art (https://smart.servier.com), courtesy of Servier, under a Creative Commons Attribution 3.0 Unported License available at https://creativecommons.org/licenses/by/3.0/
Pulse wave velocity
Pulse wave velocity (PWV) is a measure of arterial diameter, pulse pressure, and pulsatile diameter change, which makes it an indicator of arterial distensibility. The aortic PWV is calculated as the difference in the travel time of the pulse waves between two different recording sites and the heart, divided by the travel distance covered by the pulse wave [31]. PWV was found to be elevated in patients with PsA compared to controls [32, 33], even in the early stages of the disease, and this increase was even more pronounced in those with long-term disease (8.2 ± 0.8 vs 6.8 ± 1.0 m/s, p < 0.001) [34]. The burden of inflammation over time was also revealed since PWV was related to erythrocyte sedimentation rate (ESR) and CRP levels, as well as DAS28 and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [35]. A higher PWV was associated with increased arterial stiffness and higher CV risk [36]. It was significantly related to a higher systolic and diastolic blood pressure, as well as a higher body mass index, all of which are possible causes of reduced arterial distensibility prevalent in patients with PsA [32, 37]. Furthermore, a different study suggested that TNFis may improve arterial stiffness as measured by PWV. However, the use of a mixed cohort of patients with RA, AS, and PsA makes it challenging to draw disease-specific conclusions [38].
Vascular remodeling in PsA
Carotid intima-media thickness
Carotid intima-media thickness (cIMT) is considered a sensitive marker of subclinical ASCVD that can be measured using a non-invasive B-mode ultrasound [39, 40]. It has been evaluated in patients with PsA with mixed results. Several studies found significantly increased cIMT both in patients with and without CVRF [41,42,43,44]. Smaller but still significant increases were observed in the general population of PsO, who showed higher cIMT than a subgroup with PsA [45]. Elevated cIMT is an independent risk factor aggravated by the coexistence of common comorbidities such as older age, body mass index, waist-to-hip ratio, systolic and diastolic blood pressure, and the presence of diabetes. All were positively correlated with cIMT, along with some disease-related parameters such as duration of disease and activity (ESR, CRP), age of onset, duration of skin and joint manifestations. Although in some studies cIMT levels were not different between the PsA and control groups [11, 19], it is speculated that this difference stemmed from the increased use of anti-TNF treatment. To our knowledge, there is still no targeted research to support this claim [21].
Carotid plaques
One of the most important indicators of subclinical ASCVD is the burden of carotid plaques. It can be measured using a carotid duplex ultrasound and is defined as a localized thickening of the arterial wall [46]. Patients with PsA have a higher prevalence of carotid plaques than the general population [44], especially in older people or patients with a history of smoking, hypertension, high levels of triglycerides, and high levels of low-density lipoprotein cholesterol. Studies have also shown a possible risk of rapid progression from unilateral to bilateral plaques and the development of new plaques in less than 2 years from the initial screening among patients with CVRF [47]. Thus, carotid plaque burden (CPB) can be a predictor of future CV events in PsA patients with subclinical atherosclerosis [46, 48]. It is independent of Disease Activity in Psoriasis (DAPSA) and traditional CVRF, and is associated with a threefold increase in the risk of developing CV events in the PsA population [46]. Patients with plaques have a longer duration of the disease and more swollen joints, although they share a similar CV risk profile with the plaque-free group [49]. Carotid total plaque area (CTPA) shows the sum of the extent of the carotid plaques and is likely a better surrogate marker than carotid intima-media thickness (cIMT) alone. Interestingly, it was found to be higher in patients with PsA compared to patients with PsO without arthritis. It was associated with an increase in the intensity of inflammation and in the duration of PsA [50]. DAPSA was also found to be an independent predictor of CV events with better sensitivity than a tender or swollen joint count [46]. In addition to carotid plaques, the burden of coronary plaques and the prevalence of CAD in PsA should also be considered. It is measured using coronary computed tomography angiography (CCTA), a non-invasive method comparable to invasive angiography that offers a quantitative and qualitative evaluation of stenotic and non-stenotic coronary plaque burden (coPB) with high precision [51, 52]. Coronary plaques are considered to be at higher risk when they are noncalcified or mixed, while calcified plaques (CP) are deemed less vulnerable. All are prevalent in PsA even when they do not cause vessel obstruction or symptomatic disease [53]. Shen et al. found in their study that 60% of patients with PsA had at least one type of plaque. Compared to controls, they had a two to threefold increase in prevalence of all types of plaques, particularly mixed plaques/noncalcified plaques (MP/NCP), which were associated with longer disease duration. Each additional year of exposure to inflammation was estimated to increase the risk of developing MP/NCP by 6%. Patients with PsA were also more likely to have three-vessel disease, obstructive lesions, and a higher segment involvement score (SIS) than controls, all indicating a higher coPB [54]. It is believed that SIS has the capability to predict major adverse cardiac events in asymptomatic patients with an initially higher risk of CAD [51]. No association was demonstrated between carotid and coronary plaque; however, mean and maximum cIMT were significantly associated with the latter [54]. In a related study, the same finding was reported, indicating that although CPB may not be a precise marker of CAD, an increase in mean cIMT was an independent explanatory variable associated with the disease [48]. These data suggest that carotid ultrasound evaluation may be a reasonable tool for CV risk assessment in asymptomatic patients, certainly better than traditional risk scores, however, not as good as CCTA [55]. A major advantage of CCTA is that it can detect the non-stenotic burden of ASCVD, which is present in patients well before the diagnosis of CAD. A study by Szentepery et al. compared patients with PsA but without CAD symptoms with matched controls. They found that PsA was associated with a higher number and extensiveness of coronary plaques, particularly of mixed type, however, there was no difference in CP and NCP. Interestingly, plaques were predominantly located in LAD coronary segments, which are generally associated with a worse prognosis. Furthermore, lipid-rich MPs are more at risk than CP, they are more often associated with thin-cap fibroatheromas, making them more prone to rupture. They are more prevalent in acute CAD compared to chronic disease. This might explain the relationship between PsA and cardiac events. To further strengthen this link, the SIS results, the segment stenosis score (SSS), and the total plaque volume (TPV) were higher in the PsA group and were correlated with measures of disease activity such as the maximum swollen joint count, maximum ESR rate, and CRP levels. Furthermore, age, disease duration, and plasma glucose level were independent predictors of a higher plaque burden in PsA. This suggests that minimizing disease activity, but only combined with optimal metabolic control, would be beneficial in preventing CV events. However, in this study, CAD was independent of the presence of metabolic syndrome in PsA. CoPB was not significantly higher in patients with characteristics of metabolic syndrome, as there was no difference in SIS, SSS, TPV, and type of plaque. TPV was associated with a diagnosis of PsA, but not with metabolic syndrome [51].
Comparison to RA
RA is a multifactorial autoimmune disease that primarily affects the synovial joints, but also the extraarticular organs. Although both RA and PsA share synovitis as their hallmark feature, they vary in clinical presentation and the details of treatment.
Endothelial dysfunction in microcirculation in RA
A meta-analysis performed by Erre et al. reported that CFR is significantly lower in patients with rheumatic diseases in general, suggesting analogous results between PsA and RA [56]. A study of patients with early RA that included 25 individuals with an average duration of 6.24 months revealed a decrease in CFR compared to healthy controls. It depicts a dysfunction of the coronary microcirculation even in the early stages of the disease [57]. CFR has also been found to be reduced in established RA in several studies [58,59,60,61]. Moreover, it was observed that CFR was inversely correlated with disease activity as measured by DAS 28, disease duration, and CRP [61]. Another study showed that coronary microvascular dysfunction, defined as CFR < 2, was associated with an increased risk of all-cause mortality in patients with RA and diabetes. Patients with coronary microvascular dysfunction were also more likely to die from cardiovascular disease. A significant decrease in CFR is similar to that in patients with PsA [60]. In a study involving nailfold capillaroscopy (NFC), a decreased capillary density was observed in patients with RA compared to the control group. The study revealed a negative correlation with CRP and PWV, while showing a positive correlation with HDL-C and cardiac index. Importantly, capillary density was also significantly associated with CVR calculated using the Framingham Risk Score (FRS). This implies that NFC could act as an important tool for estimating CVR in RA patients [62]. No similar associations were found with the NFC findings in PsA. Abnormal findings in NFC are common in patients with PsA and RA and both these groups are characterized by similar morphological abnormalities, including tortuosity and reduced capillary density, but, in contrast to RA, their significance for overall ED and CVR in PsA remains unknown.
Endothelial dysfunction in macrocirculation in RA
FMD has been widely investigated in patients with RA and its impairment has been observed in numerous studies [63]. One reported that even people with early RA have altered FMD when compared to controls, suggesting that ED can be detected regardless of the short course of the disease [64]. In terms of factors that may be associated with ED measured by this parameter, it was revealed that lower values of FMD [%] were correlated with certain shared epitope alleles: HLA-DRB1*04 and HLA-DRB1*0404. Furthermore, the same research evaluated endothelium-independent vasodilation after nitroglycerin administration, which was not significantly different between the study and the control group, further implicating impaired NO release as a major contributor to the vascular alterations present in patients with RA [65]. Other researchers found an association between decreased FMD and CRP levels, suggesting that inflammation plays an important role in people with ED in RA [66, 67]. Additionally, one of these studies found an association between FMD and CRP [67]. Interestingly, CCR5Δ32 deletion appears to be a protective factor for ED measured by FMD. The CCR5 receptor is present in T lymphocytes and antigen-presenting cells, including macrophages or dendritic cells, and is involved in trafficking and activation [68, 69]. Notably, it is also expressed in vascular smooth muscle cells [70]. Rodrguez-Rodrguez et al. reported in their work that patients with RA who are carriers of this deletion presented significantly higher FMD values compared to other patients, implying that the CCR5 molecule may play a role in the development of ASCVD in RA [71].
Arterial stiffness in RA
A study by Becetti et al. showed that patients with RA had elevated AIx compared to controls. These values were associated with albuminuria, as assessed by the urinary albumin-to-creatinine ratio, which was also directly correlated with vascular cell adhesion molecule 1 (VCAM-1) levels, an indicator of endothelial activation, and inversely with interleukin-10 levels, an anti-inflammatory cytokine. This suggests that not only hypertension or diabetes but also albuminuria could be another marker of systemic vascular damage in RA [72]. Noteworthy data depicted a direct correlation between aortic PWV and epicardial adipose tissue in patients with RA [73]. Epicardial adipose tissue is a layer of fat located between the surface of the heart and the visceral pericardium [74]. Physiologically, it balances the amount of free fatty acids within the myocardium, plays a role in thermoregulation, and mechanically protects the autonomic nerves and ganglia that innervate the heart muscle [75]. However, in pathological conditions such as obesity, due to its proximity to the myocardium and its shared microcirculation with this tissue, it is believed to play a specific role in the pathogenesis of CAD through paracrine and so-called vasocrine activity, via the coronary vasa vasorum. It releases numerous factors, such as pro-inflammatory cytokines, pro-fibrotic agents, or metalloproteinases, which contribute to the progression of CV disease [76, 77]. Additionally, increased arterial stiffness has been found to alter left ventricular function. One study found an association between elevated PWV and increased filling pressure of the left ventricle [78], while another study showed that the left ventricular myocardial performance index, an indicator of systolic and diastolic functions, was positively correlated with carotid-femoral PWV and AIx corrected for heart rate [79]. These studies suggest that aortic stiffness could act as an indicator of not only vascular damage but also heart muscle involvement, thereby increasing the CVR in RA patients. A study that evaluated PWV in patients with early RA and PsA revealed that both groups had a higher PWV compared to controls, but it was significantly higher in individuals with RA (PsA vs RA vs HC, mean ± SD, 6.42 ± 1.39 vs 7.91 ± 1.93 vs 5.11 ± 0.83 m/s, p < 0.001; PsA vs RA, p = 0.009) [80]. This suggests that arterial stiffness increases in the early stages of both types of arthritis, but it seems to be more pronounced in RA.
Vascular remodeling
Increased values of cIMT have been frequently observed among patients with RA compared to controls [81, 82]. Some studies have found associations between this marker and indices of inflammation or disease activity, such as CRP [83,84,85] and DAS28 [86, 87]. In a study conducted in patients with early RA, cIMT was increased but still within the normal range compared to healthy controls [57], while the other research showed an increase in cIMT in early RA. However, it revealed that this marker did not differ significantly between early PsA patients and healthy controls [80]. These results suggest that vascular remodeling is present in RA even in the early stages of the disease, whereas in PsA it is less apparent. Patients with RA had significantly increased cIMT, which was also positively associated with Homeostatic Model Assessment for Insulin Resistance (HOMA2-IR). It indicates that impaired insulin sensitivity could be another potential risk factor for the development of ASCVD in patients with RA [86]. Another study found that an increase in cIMT in RA was correlated with higher levels of highly pro-inflammatory CD4+/CD28+ T lymphocytes and higher expression of the fractalkine receptor (CX3CR1) on them, which is responsible for interaction with the chemokine CX3CL1 [87]. Increased numbers of these cells have been observed among patients with unstable angina compared to patients with stable angina, suggesting their role in the infiltration of unstable plaques [88, 89]. In addition to its role as a chemotactic factor, CX3CR1 can also act as an adhesion molecule for leukocytes, expressed in activated endothelial cells [90]. Therefore, upregulation of the fractalkine receptor may allow T cells to infiltrate the tissue and cause its damage, accelerating the atherosclerotic process [87]. In other relevant research FMD combined with cIMT was compared among individuals with RA, PsA, and controls. Patients with CV disease or pre-existing traditional CVRF were excluded from the investigation. In terms of FMD, the RA and PsA groups had lower values of this marker compared to controls. However, cIMT was significantly elevated only in the RA group, while the result in the PsA group was similar to healthy controls. Furthermore, carotid plaques, defined as thickening of intima-media > 1.0 mm, were detected only in the RA group, with no such cases in the PsA and control groups. The researchers suggested that the reason for this outcome might be the greater use of anti-TNF treatment in the PsA group, which has been shown to significantly reduce cIMT in psoriatic patients [91, 92], thus improving vascular remodeling in this group [21].
Carotid plaques
Similarly to PsA, patients with RA are more susceptible to develop carotid plaques than the general population [93, 94]. Both CPB and cIMT are significantly associated with CVRF, consequently stroke and myocardial infarction, which can be avoided by identifying asymptomatic ASCVD in RA. [95] Evans et al. found an association between the presence of unilateral carotid plaque at baseline and an increased risk of acute coronary syndrome (ACS). The incidence of ACS could even increase fourfold if the plaques were present in both internal carotid arteries [96]. The impact of ASCVD on ACS in patients with RA may be an indication for the use of carotid ultrasound when evaluating CVR [97]. A study by Karpouzas et al. used computed tomography angiography to establish that noncalcified coronary plaque was found in 54% of afflicted arteries of asymptomatic RA patients compared to 21% in controls. Additionally, the plaque was more extensive in RA patients [98]. Furthermore, significantly elevated CRP levels have an impact on the appearance of unstable coronary plaque in CCTA [97, 99]. Increased CPB, CTPA, and coPB are associated with an elevated risk of CV events and ACS in patients with RA and, to a lesser extent, in PsA.
Discussion
As observed in other studies, patients with PsO as well as patients with PsA have increased cardiovascular risk defined as a greater likelihood of major adverse cardiac events and a higher incidence of cardiometabolic comorbidities such as arterial hypertension, hypercholesterolemia, diabetes, obesity, metabolic syndrome, or non-alcoholic fatty liver disease compared to the general population. There is an unmet need for improvement in the primary or secondary prevention of cardiovascular diseases in patients with PsO and PsA by implementing the appropriate pharmaceutical interventions and adjusting risk stratification. Research shows that the use of traditional risk algorithms underestimates the likelihood of future CV events in patients with PsA [46, 48]. The widely used Framingham Risk Score (FRS) takes into account age, sex, smoking, hypertension, total cholesterol, and HDL-C but overlooks the inflammatory burden, diversity of PsA clinical phenotype, and a vast range of comorbidities. When comparing FRS and Systematic Coronary Risk Evaluation (SCORE) with cIMT and CTPA, an alarming number of patients must be reclassified into higher-risk groups based on ultrasound results. These imaging findings improve risk stratification, and the presence of carotid plaques is even more predictive than cIMT. The DAPSA score was associated with reclassification when using the SCORE algorithm, further demonstrating the influence of chronic inflammation and active disease [100, 101]. The European Alliance of Associations for Rheumatology (EULAR) recommends a multiplication factor of 1.5 for risk scores in RA patients only. Still, research suggests that similar considerations should apply to PsA [102]. This is particularly noteworthy because, upon comparing both groups, CTPA and the severity of subclinical ASCVD are even higher in PsA. These results are independent of traditional CVRF and show how suboptimal traditional scales are for stratifying CVR in PsA [50]. It has been shown that the progression of subclinical ASCVD can be slowed by achieving sMDA. There are two other measures of disease activity that should be considered in the context of reducing the general inflammatory burden. These are the DAPSA index, which includes the number of swollen and tender joints, CRP, visual analogue scale for pain as well as disease activity assessment, and PASDAS, which similarly to sMDA, includes peripheral joint, but also skin, and enthesitis domains. Sustained low disease activity according to PASDAS was shown to prevent carotid ASCVD and progression of arterial stiffness. The same was not achieved for DAPSA. This difference may suggest that some components, such as skin manifestation, may influence the severity of the disease more than previously anticipated [30]. Interestingly, a promising anti-TNF therapy failed to achieve the anti-atherogenic effect apart from the improvement in lipid profiles [103]. The role of this work was not to assess the benefits of anti-TNF therapy, although it is speculated that this treatment may have been, after all, beneficial in different ways and may have accounted for the variability in the study results, including studies comparing RA and PsA. Data suggest that indicators of arterial stiffness, including AIx and PWV, improve during TNFi treatment and the lower prevalence of vascular remodeling expressed as increased cIMT in PsA suggests the efficacy of this therapy.
Conclusions
Evidence from the literature shows that PsA, similarly to RA, leads to ED over the course of the disease. ED plays an important role in the pathophysiology of cardiometabolic diseases. PsA has been found to be associated with the accelerated atherosclerosis process, which may also be related to the prevalence of metabolic syndrome. PsA and other forms of arthritis or autoimmune inflammatory diseases should be further investigated to determine the extent of their negative impact on the endothelium, and consequently the significance of their role in the development of ASCVD. Patients burdened with inflammatory diseases, especially arthritis, should receive special attention during the assessment of cardiovascular risk. Minimisation of PsA and RA activity, tight control of classic CVRF, as well as early detection of asymptomatic cases of ASCVD, could be beneficial in preventing CV events and limiting CV mortality. The EULAR recommendation to multiply CVR score by 1.5 in patients with RA should also be considered with respect to patients with PsA, whose CTPA and consequently the severity of subclinical ASCVD is even higher than in patients with RA. Finally, anti-TNF treatment appears to have a beneficial effect on endothelial function in patients with PsA by reducing markers of inflammation and cIMT, thereby improving vascular remodeling, which, in contrast, is more pronounced in patients with RA. However, further research is needed, as long-term reversal of ED and atherosclerosis progression is not easily attainable, even at the age of biologic and targeted synthetic drugs used in RA and PsA.
Abbreviations
- ACS:
-
Acute coronary syndrome
- ADMA:
-
Asymmetric dimethylarginine
- AIx:
-
Augmentation index
- ASCVD:
-
Atherosclerotic cardiovascular disease
- BASDAI:
-
Bath Ankylosing Spondylitis Disease Activity Index
- CAD:
-
Coronary artery disease
- CCR:
-
CC chemokine receptor
- CCTA:
-
Coronary computed tomography angiography
- CFR:
-
Coronary flow reserve
- cIMT:
-
Carotid intima-media thickness
- coPB:
-
Coronary plaque burden
- CP:
-
Calcified plaques
- CPB:
-
Carotid plaque burden
- CTPA:
-
Carotid total plaque area
- CV:
-
Cardiovascular
- CVR:
-
Cardiovascular risk
- CVRF:
-
Cardiovascular risk factors
- DAPSA:
-
Disease Activity in Psoriatic Arthritis
- DAS28:
-
Disease Activity Score in 28 Joints
- DMARDs:
-
Disease-modifying anti-rheumatic drugs
- EAT:
-
Epicardial adipose tissue
- ED:
-
Endothelial dysfunction
- ESR:
-
Erythrocyte sedimentation rate
- EULAR:
-
European League Against Rheumatism
- FMD:
-
Flow-mediated dilatation
- FRS:
-
Framingham Risk Score
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- hs-CRP:
-
High-sensitivity C-reactive protein
- IL:
-
Interleukin
- IR:
-
Insulin resistance
- MP:
-
Mixed plaques
- NCP:
-
Noncalcified plaques
- NFC:
-
Nailfold capillaroscopy
- NMD:
-
Nitroglycerin-mediated dilation
- NO:
-
Nitric oxide
- PASDAS:
-
Psoriatic Arthritis Disease Activity Score
- PASI:
-
Psoriasis Area and Severity Index
- PsA:
-
Psoriatic arthritis
- PsO:
-
Psoriasis
- PWV:
-
Pulse-wave velocity
- RA:
-
Rheumatoid arthritis
- SCORE:
-
Systemic Coronary Risk Evaluation
- SIS:
-
Segment involvement score
- sMDA:
-
Sustained minimal disease activity
- SSS:
-
Segment stenosis score
- TNF:
-
Tumor necrosis factor
- TNFi:
-
Tumor necrosis factor inhibitor
- TPV:
-
Total plaque volume
References
Ritchlin CT, Colbert RA, Gladman DD (2017) Psoriatic arthritis. N Engl J Med 376:957–970. https://doi.org/10.1056/NEJMra1505557
Mease PJ, Armstrong AW (2014) Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs 74:423–441. https://doi.org/10.1007/s40265-014-0191-y
Coates LC, Helliwell PS (2017) Psoriatic arthritis: state of the art review. Clin Med 17:65–70. https://doi.org/10.7861/clinmedicine.17-1-65
Ciocon DH, Kimball AB (2007) Psoriasis and psoriatic arthritis: separate or one and the same? Br J Dermatol 157:850–860. https://doi.org/10.1111/j.1365-2133.2007.08148.x
Atzeni F, Turiel M, Boccassini L et al (2011) Cardiovascular involvement in psoriatic arthritis. Reumatismo 63:148–154. https://doi.org/10.4081/reumatismo.2011.148
Deanfield J, Donald A, Ferri C et al (2005) Endothelial function and dysfunction. Part I: methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens 23:7–17. https://doi.org/10.1097/00004872-200501000-00004
di Minno MND, Ambrosino P, Lupoli R et al (2015) Cardiovascular risk markers in patients with psoriatic arthritis: a meta-analysis of literature studies. Ann Med 47:346–353. https://doi.org/10.3109/07853890.2015.1031822
Han C, Robinson DW, Hackett MV et al (2006) Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 33:2167–2172
Jamil M, Aslam R, Patel A et al (2021) Prevalence and extent of subclinical atherosclerosis and associated cardiovascular risk factors in adult patients with psoriatic arthritis: a systematic review. Cureus. https://doi.org/10.7759/cureus.16853
Gasparyan AY, Ayvazyan L, Blackmore H et al (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417. https://doi.org/10.1007/s00296-011-1999-3
Atzeni F, Sarzi-Puttini P, Sitia S et al (2011) Coronary flow reserve and asymmetric dimethylarginine levels: new measurements for identifying subclinical atherosclerosis in patients with psoriatic arthritis. J Rheumatol 38:1661–1664. https://doi.org/10.3899/jrheum.100893
Mahfouz RA, Mostafa T, Fahmy DS (2014) Impact of the neutrophil-to-lymphocyte ratio on coronary flow reserve and incipient myocardial dysfunction in patients with psoriatic arthritis. J Arthritis 03:1–5. https://doi.org/10.4172/2167-7921.1000119
Piaserico S, Osto E, Famoso G et al (2019) Long-term prognostic value of coronary flow reserve in psoriasis patients. Atherosclerosis 289:57–63. https://doi.org/10.1016/j.atherosclerosis.2019.08.009
Ikonomidis I, Pavlidis G, Katogiannis K et al (2022) Differences in coronary flow reserve and flow-mediated dilation between plaque psoriasis and psoriatic arthritis. Eur Heart J 43:2643. https://doi.org/10.1093/eurheartj/ehac544.2643
Murdaca G, Bezante G, Penza E et al (2016) Endothelial dysfunction in psoriatic arthritis: evaluation by endothelial-dependent flow-mediated dilation and coronary flow reserve. Ann Rheum Dis 75:600. https://doi.org/10.1136/annrheumdis-2016-eular.5725
Piaserico S, Papadavid E, Cecere A et al (2023) Coronary microvascular dysfunction in asymptomatic patients with severe psoriasis. J Invest Dermatol 143:1929-1936.e2. https://doi.org/10.1016/j.jid.2023.02.037
Piaserico S, Osto E, Famoso G et al (2016) Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis 251:25–30. https://doi.org/10.1016/j.atherosclerosis.2016.05.036
Moroni L, Selmi C, Angelini C et al (2017) Evaluation of endothelial function by flow-mediated dilation: a comprehensive review in rheumatic disease. Arch Immunol Ther Exp (Warsz) 65:463–475. https://doi.org/10.1007/s00005-017-0465-7
Yilmazer B, Sahin T, Unlu BÖ et al (2015) Investigation of subclinical atherosclerosis in psoriatic arthritis patients with minimal disease activity. Rheumatol Int 35:1385–1392. https://doi.org/10.1007/s00296-015-3228-y
Gonzalez-Juanatey C, Llorca J, Miranda-Filloy JA et al (2007) Endothelial dysfunction in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Care Res (Hoboken) 57:287–293. https://doi.org/10.1002/art.22530
Bilgen ŞA, Kalyoncu U, Erden A et al (2018) Assessment of subclinical atherosclerosis in psoriatic arthritis patients without clinically overt cardiovascular disease or traditional atherosclerosis risk factors. Turk Kardiyol Dern Ars 46:358–365. https://doi.org/10.5543/tkda.2018.36169
Turoňová L, Kubejová K, Vorčáková K et al (2018) Endothelial dysfunction in children with juvenile psoriatic arthritis. Acta Medica (Hradec Kralove) 61:79–85. https://doi.org/10.14712/18059694.2018.122
Sharma A, Reddy MH, Sharma K et al (2014) Study of endothelial dysfunction in patients of psoriatic arthritis by flow mediated and nitroglycerine mediated dilatation of brachial artery. Int J Rheum Dis 19:300–304. https://doi.org/10.1111/1756-185X.12336
Verma S, Wang CH, Li SH et al (2002) A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 106:913–919. https://doi.org/10.1161/01.CIR.0000029802.88087.5E
Dimitroulas T, Sandoo A, Kitas GD (2012) Asymmetric dimethylarginine as a surrogate marker of endothelial dysfunction and cardiovascular risk in patients with systemic rheumatic diseases. Int J Mol Sci 13:12315–12335. https://doi.org/10.3390/ijms131012315
Shang Q, Tam LS, Sanderson JE et al (2012) Increase in ventricular-arterial stiffness in patients with psoriatic arthritis. Rheumatology (Oxford) 51:2215–2223. https://doi.org/10.1093/rheumatology/kes213
Patschan D, Sugiarto N, Henze E et al (2018) Early endothelial progenitor cells and vascular stiffness in psoriasis and psoriatic arthritis. Eur J Med Res 23:56. https://doi.org/10.1186/s40001-018-0352-7
Angel K, Provan SA, Hammer HB et al (2011) Changes in arterial stiffness during continued infliximab treatment in patients with inflammatory arthropathies. Fundam Clin Pharmacol 25:511–517. https://doi.org/10.1111/j.1472-8206.2010.00872.x
Cheng IT, Shang Q, Li EK et al (2019) Effect of achieving minimal disease activity on the progression of subclinical atherosclerosis and arterial stiffness: a prospective cohort study in psoriatic arthritis. Arthritis Rheumatol 71:271–280. https://doi.org/10.1002/art.40695
Cheng IT, Li EK, Wong PC et al (2020) Treat to target and prevention of subclinical atherosclerosis in psoriatic arthritis—which target should we choose? Rheumatology (Oxford) 59:2881–2892. https://doi.org/10.1093/rheumatology/keaa025
Brezinski EA, Follansbee MR, Armstrong EJ et al (2014) Endothelial dysfunction and the effects of TNF inhibitors on the endothelium in psoriasis and psoriatic arthritis: a systematic review. Curr Pharm Des 20:513–528. https://doi.org/10.2174/138161282004140213123852
Soy M, Yildiz M, Sevki Uyanik M et al (2009) Susceptibility to atherosclerosis in patients with psoriasis and psoriatic arthritis as determined by carotid–femoral (aortic) pulse-wave velocity measurement. Rev Esp Cardiol 62:96–99. https://doi.org/10.1016/s1885-5857(09)71520-8
Triantafyllias K, Liverakos S, Klonowski A et al (2022) High cardiovascular risk in patients with psoriatic arthritis: evaluation of macroangiopathy and its predictors by aortic pulse wave velocity. Ann Rheum Dis 81:869–870. https://doi.org/10.1136/annrheumdis-2022-eular.2670
Costa L, Caso F, D’Elia L et al (2012) Psoriatic arthritis is associated with increased arterial stiffness in the absence of known cardiovascular risk factors: a case control study. Clin Rheumatol 31:711–715. https://doi.org/10.1007/s10067-011-1892-1
Shen J, Shang Q, Li EK et al (2015) Cumulative inflammatory burden is independently associated with increased arterial stiffness in patients with psoriatic arthritis: a prospective study. Arthritis Res Ther 17:75. https://doi.org/10.1186/s13075-015-0570-0
Imura T, Yamamoto K, Kanamori K et al (1986) Non-invasive ultrasonic measurement of the elastic properties of the human abdominal aorta. Cardiovasc Res 20:208–214. https://doi.org/10.1093/cvr/20.3.208
Yildiz M (2010) Arterial distensibility in chronic inflammatory rheumatic disorders. Open Cardiovasc Med J 4:83–88. https://doi.org/10.2174/1874192401004020083
Angel K, Provan SA, Gulseth HL et al (2010) Tumor necrosis factor-α antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension 55:333–338. https://doi.org/10.1161/HYPERTENSIONAHA.109.143982
Ludwig M, Petzinger-Kruthoff AV, Buquoy MV et al (2003) Intima-media-dicke der karotisarterien: früher indikator für arteriosklerose und therapeutischer endpunkt. Ultraschall Med 24:162–174. https://doi.org/10.1055/s-2003-40058
Nezu T, Hosomi N, Aoki S et al (2016) Carotid intima-media thickness for atherosclerosis. J Atheroscler Thromb 23:18–31. https://doi.org/10.5551/jat.31989
Kimhi O, Caspi D, Bornstein NM et al (2007) Prevalence and risk factors of atherosclerosis in patients with psoriatic arthritis. Semin Arthritis Rheum 36:203–209. https://doi.org/10.1016/j.semarthrit.2006.09.001
Tam LS, Shang Q, Li EK et al (2008) Subclinical carotid atherosclerosis in patients with psoriatic arthritis. Arthritis Care Res (Hoboken) 59:1322–1331. https://doi.org/10.1002/art.24014
Garg N, Krishan P, Syngle A (2016) Atherosclerosis in psoriatic arthritis: a multiparametric analysis using imaging technique and laboratory markers of inflammation and vascular function. Int J Angiol 25:222–228. https://doi.org/10.1055/s-0036-1584918
Eder L, Zisman D, Barzilai M et al (2008) Subclinical atherosclerosis in psoriatic arthritis: a case-control study. J Rheumatol 35:877–882
Fang N, Jiang M, Fan Y (2016) Association between psoriasis and subclinical atherosclerosis. Medicine (Baltimore) 95:e3576. https://doi.org/10.1097/MD.0000000000003576
Lam SHM, Cheng IT, Li EK et al (2020) DAPSA, carotid plaque and cardiovascular events in psoriatic arthritis: a longitudinal study. Ann Rheum Dis 79:1320–1326. https://doi.org/10.1136/annrheumdis-2020-217595
Lucke M, Messner W, Kim ESH et al (2016) The impact of identifying carotid plaque on addressing cardiovascular risk in psoriatic arthritis. Arthritis Res Ther 18:178. https://doi.org/10.1186/s13075-016-1074-2
Cheng IT, Wong KT, Li EK et al (2020) Comparison of carotid artery ultrasound and Framingham risk score for discriminating coronary artery disease in patients with psoriatic arthritis. RMD Open 6:e001364. https://doi.org/10.1136/rmdopen-2020-001364
Cheng IT, Meng H, Li M et al (2022) Serum calprotectin level is independently associated with carotid plaque presence in patients with psoriatic arthritis. Front Med (Lausanne) 9:932696. https://doi.org/10.3389/fmed.2022.932696
Eder L, Jayakar J, Shanmugarajah S et al (2013) The burden of carotid artery plaques is higher in patients with psoriatic arthritis compared with those with psoriasis alone. Ann Rheum Dis 72:715–720. https://doi.org/10.1136/annrheumdis-2012-201497
Szentpetery A, Healy GM, Brady D et al (2018) Higher coronary plaque burden in psoriatic arthritis is independent of metabolic syndrome and associated with underlying disease severity. Arthritis Rheumatol 70:396–407. https://doi.org/10.1002/art.40389
Miller JM, Rochitte CE, Dewey M et al (2008) Diagnostic performance of coronary angiography by 64-row CT. N Eng J Med 359:2324–2336. https://doi.org/10.1056/NEJMoa0806576
Tinggaard AB, Hjuler KF, Andersen IT et al (2021) Prevalence and severity of coronary artery disease linked to prognosis in psoriasis and psoriatic arthritis patients: a multi-centre cohort study. J Intern Med 290:693–703. https://doi.org/10.1111/joim.13311
Shen J, Wong KT, Cheng IT et al (2017) Increased prevalence of coronary plaque in patients with psoriatic arthritis without prior diagnosis of coronary artery disease. Ann Rheum Dis 76:1237–1244. https://doi.org/10.1136/annrheumdis-2016-210390
Haroon M, Szentpetery A, Dodd JD et al (2018) Modifications of cardiovascular risk scores, but not standard risk scores, improve identification of asymptomatic coronary artery disease in psoriatic arthritis. J Rheumatol 45:1329–1330. https://doi.org/10.3899/jrheum.180299
Erre GL, Buscetta G, Paliogiannis P et al (2018) Coronary flow reserve in systemic rheumatic diseases: a systematic review and meta-analysis. Rheumatol Int 38:1179–1190. https://doi.org/10.1007/s00296-018-4039-8
Turiel M, Atzeni F, Tomasoni L et al (2009) Non-invasive assessment of coronary flow reserve and ADMA levels: a case-control study of early rheumatoid arthritis patients. Rheumatology (Oxford) 48:834–839. https://doi.org/10.1093/rheumatology/kep082
Ciftci O, Yilmaz S, Topcu S et al (2008) Impaired coronary microvascular function and increased intima-media thickness in rheumatoid arthritis. Atherosclerosis 198:332–337. https://doi.org/10.1016/j.atherosclerosis.2007.11.013
Kakuta K, Dohi K, Sato Y et al (2016) Chronic inflammatory disease is an independent risk factor for coronary flow velocity reserve impairment unrelated to the processes of coronary artery calcium deposition. J Am Soc Echocardiogr 29:173–180. https://doi.org/10.1016/j.echo.2015.09.001
Liao KP, Huang J, He Z et al (2021) Coronary microvascular dysfunction in rheumatoid arthritis compared to diabetes mellitus and association with all-cause mortality. Arthritis Care Res (Hoboken) 73:159–165. https://doi.org/10.1002/acr.24108.Coronary
Recio-Mayoral A, Mason JC, Kaski JC et al (2009) Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J 30:1837–1843. https://doi.org/10.1093/eurheartj/ehp205
Anyfanti P, Gkaliagkousi E, Triantafyllou A et al (2018) Dermal capillary rarefaction as a marker of microvascular damage in patients with rheumatoid arthritis: association with inflammation and disorders of the macrocirculation. Microcirculation 25:e12451. https://doi.org/10.1111/micc.12451
Xu SZ, Wang P, Guan SY et al (2017) Decreased flow-mediated dilatation in patients with rheumatoid arthritis: a meta-analysis. Postgrad Med J 93:260–265. https://doi.org/10.1136/postgradmedj-2016-134068
Chatterjee Adhikari M, Guin A, Chakraborty S et al (2012) Subclinical atherosclerosis and endothelial dysfunction in patients with early rheumatoid arthritis as evidenced by measurement of carotid intima-media thickness and flow-mediated vasodilatation: an observational study. Semin Arthritis Rheum 41:669–675. https://doi.org/10.1016/j.semarthrit.2011.08.003
Gonzalez-Juanatey C, Testa A, Garcia-Castelo A et al (2003) HLA-DRB1 status affects endothelial function in treated patients with rheumatoid arthritis. Am J Med 114:647–652. https://doi.org/10.1016/S0002-9343(03)00133-5
Arosio E, De MS, Rigoni A et al (2007) Forearm haemodynamics, arterial stiffness and microcirculatory reactivity in rheumatoid arthritis. J Hypertens 25:1273–1278. https://doi.org/10.1097/HJH.0b013e3280b0157e
Vaudo G, Marchesi S, Gerli R et al (2004) Endothelial dysfunction in young patients with rheumatoid arthritis and low disease activity. Ann Rheum Dis 63:31–35. https://doi.org/10.1136/ard.2003.007740
Lederman MM, Penn-Nicholson A, Cho M et al (2009) Biology of CCR5 and its role in HIV infection and treatment. JAMA 296:815–826. https://doi.org/10.1001/jama.296.7.815
Oppermann M (2004) Chemokine receptor CCR5: insights into structure, function, and regulation. Cell Signal 16:1201–1210. https://doi.org/10.1016/j.cellsig.2004.04.007
Schecter AD, Calderon TM, Berman AB et al (2000) Human vascular smooth muscle cells possess functional CCR5. J Biol Chem 275:5466–5471. https://doi.org/10.1074/jbc.275.8.5466
Rodríguez-Rodríguez L, González-Juanatey C, García-Bermúdez M et al (2011) CCR5Δ32 variant and cardiovascular disease in patients with rheumatoid arthritis: a cohort study. Arthritis Res Ther 13:R133. https://doi.org/10.1186/ar3444
Becetti K, Oeser A, Ormseth MJ et al (2015) Urinary albumin excretion is increased in patients with rheumatoid arthritis and associated with arterial stiffness. J Rheumatol 42:593–598. https://doi.org/10.3899/jrheum.141295
Petra CV, Albu A, Pamfil C et al (2019) The relationship between epicardial adipose tissue and arterial stiffness in patients with rheumatoid arthritis. Med Ultrason 21:427–434. https://doi.org/10.11152/mu-2001
Berg G, Miksztowicz V, Morales C et al (2019) Epicardial adipose tissue in cardiovascular disease. Adv Exp Med Biol 1127:131–143. https://doi.org/10.1007/978-3-030-11488-6_9
Antonopoulos AS, Antoniades C (2017) The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles. J Physiol 595:3907–3917. https://doi.org/10.1113/JP273049
Ansaldo AM, Montecucco F, Sahebkar A et al (2019) Epicardial adipose tissue and cardiovascular diseases. Int J Cardiol 278:254–260. https://doi.org/10.1016/j.ijcard.2018.09.089
Iacobellis G (2022) Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol 19:593–606. https://doi.org/10.1038/s41569-022-00679-9
Mokotedi L, Gunter S, Robinson C et al (2019) Early wave reflection and pulse wave velocity are associated with diastolic dysfunction in rheumatoid arthritis. J Cardiovasc Transl Res 12:580–590. https://doi.org/10.1007/s12265-019-09892-3
Ilter A, Kiris A, Karkucak M et al (2016) Arterial stiffness is associated with left ventricular dysfunction in patients with rheumatoid arthritis. Clin Rheumatol 35:2663–2668. https://doi.org/10.1007/s10067-015-3163-z
Lo Gullo A, Rodríguez-Carrio J, Aragona CO et al (2018) Subclinical impairment of myocardial and endothelial functionality in very early psoriatic and rheumatoid arthritis patients: association with vitamin D and inflammation. Atherosclerosis 271:214–222. https://doi.org/10.1016/j.atherosclerosis.2018.03.004
van Sijl AM, Peters MJ, Knol DK et al (2011) Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 40:389–397. https://doi.org/10.1016/j.semarthrit.2010.06.006
Wang P, Guan S, Xu S et al (2016) Increased carotid intima-media thickness in rheumatoid arthritis: an update meta-analysis. Clin Rheumatol 35:315–323. https://doi.org/10.1007/s10067-015-3130-8
Gonzalez-Gay MA, Gonzalez-Juanatey C, Piñeiro A et al (2005) High-grade C-reactive protein elevation correlates with accelerated atherogenesis in patients with rheumatoid arthritis. J Rheumatol 32:1219–1223
Hannawi S, Haluska B, Marwick TH et al (2007) Atherosclerotic disease is increased in recent-onset rheumatoid arthritis: a critical role for inflammation. Arthritis Res Ther 9:1–9. https://doi.org/10.1186/ar2323
Pahor A, Hojs R, Gorenjak M et al (2006) Accelerated atherosclerosis in pre-menopausal female patients with rheumatoid arthritis. Rheumatol Int 27:119–123. https://doi.org/10.1007/s00296-006-0176-6
la Montagna G, Cacciapuoti F, Buono R et al (2007) Insulin resistance is an independent risk factor for atherosclerosis in rheumatoid arthritis. Diabetes Vasc Dis Res 4:130–135. https://doi.org/10.3132/dvdr.2007.031
Pingiotti E, Cipriani P, Marrelli A et al (2007) Surface expression of fractalkine receptor (CX3CR1) on CD4+/CD28− T cells in RA patients and correlation with atherosclerotic damage. Ann N Y Acad Sci 1107:32–41. https://doi.org/10.1196/annals.1381.004
Dumitriu IE, Baruah P, Finlayson CJ et al (2012) High levels of costimulatory receptors OX40 and 4–1BB characterize CD4+CD28 null T cells in patients with acute coronary syndrome. Circ Res 110:857–869. https://doi.org/10.1161/CIRCRESAHA.111.261933
Liuzzo G, Goronzy JJ, Yang H et al (2000) Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 101:2883–2888. https://doi.org/10.1161/01.cir.101.25.2883
Umehara H, Bloom ET, Okazaki T et al (2004) Fractalkine in vascular biology: from basic research to clinical disease. Arterioscler Thromb Vasc Biol 24:34–40. https://doi.org/10.1161/01.ATV.0000095360.62479.1F
Jókai H, Szakonyi J, Kontár O et al (2013) Impact of effective tumor necrosis factor-alfa inhibitor treatment on arterial intima-media thickness in psoriasis: results of a pilot study. J Am Acad Dermatol 69:523–529. https://doi.org/10.1016/j.jaad.2013.06.019
Markelova EI, Novikova DS, Korotaeva T et al (2018) Dynamics of carotid intima-media thickness, parameters of arterial stiffness and ambulatory blood pressure monitoring during therapy with inhibitor of tumor necrosis factor-alpha in patients with early psoriatic arthritis. Ration Pharmacother Cardiol 14:711–715. https://doi.org/10.20996/1819-6446-2018-14-5-711-715
Solomon DH, Reed GW, Kremer JM et al (2015) Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol 67:1449–1455. https://doi.org/10.1002/art.39098
del Rincón I, Williams K, Stern MP et al (2003) Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum 48:1833–1840. https://doi.org/10.1002/art.11078
Kobayashi H, Giles JT, Polak JF et al (2010) Increased prevalence of carotid artery atherosclerosis in rheumatoid arthritis is artery-specific. J Rheumatol 37:730–739. https://doi.org/10.3899/jrheum.090670
Evans MR, Escalante A, Battafarano DF et al (2011) Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum 63:1211–1220. https://doi.org/10.1002/art.30265
Charles-Schoeman C (2012) Cardiovascular disease and rheumatoid arthritis: an update. Curr Rheumatol Rep 14:455–462. https://doi.org/10.1007/s11926-012-0271-5
Karpouzas GA, Bui VL, Ronda N et al (2021) Biologics and atherosclerotic cardiovascular risk in rheumatoid arthritis: a review of evidence and mechanistic insights. Expert Rev Clin Immunol 17:355–374. https://doi.org/10.1080/1744666X.2021.1899809
Mal K, Kumar R, Mansoor F et al (2020) Risk of major adverse cardiovascular events in patients with rheumatoid arthritis. Cureus 12:e12246. https://doi.org/10.7759/cureus.12246
Palmou-Fontana N, Martínez-Lopez D, Corrales A et al (2020) Disease activity influences cardiovascular risk reclassification based on carotid ultrasound in patients with psoriatic arthritis. J Rheumatol 47:1344–1353. https://doi.org/10.3899/jrheum.190729
Eder L, Chandran V, Gladman DD (2013) The Framingham Risk Score underestimates the extent of subclinical atherosclerosis in patients with psoriatic disease. Ann Rheum Dis 73:1990–1996. https://doi.org/10.1136/annrheumdis
Ibáñez-Bosch R, Restrepo-Velez J, Medina-Malone M et al (2017) High prevalence of subclinical atherosclerosis in psoriatic arthritis patients: a study based on carotid ultrasound. Rheumatol Int 37:107–112. https://doi.org/10.1007/s00296-016-3617-x
Ramonda R, Puato M, Punzi L et al (2014) Atherosclerosis progression in psoriatic arthritis patients despite the treatment with tumor necrosis factor-alpha blockers: a two-year prospective observational study. Jt Bone Spine 81:421–425. https://doi.org/10.1016/j.jbspin.2014.02.005
Funding
The study was funded by the National Science Centre, Poland (project number: 2019/35/N/NZ5/04431).
Author information
Authors and Affiliations
Contributions
Conceptualization: KK, JN, MK; Methodology: KK, JN; Literature review, preparation and writing the original article: KK, JK, AS, WS, JN; Visualisation: KK, AS; Language fine-tuning: WS; Reviewing and editing the manuscript: KK, JK, AS, WS, JN, MK; Supervision: JN, MK. All authors take full responsibility for the accuracy and integrity of all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaleta, K., Krupa, J., Suchy, W. et al. Endothelial dysfunction and risk factors for atherosclerosis in psoriatic arthritis: overview and comparison with rheumatoid arthritis. Rheumatol Int (2024). https://doi.org/10.1007/s00296-024-05556-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00296-024-05556-x