Abstract
We performed a systematic review to explore existing evidence regarding the efficacy of lifestyle interventions for the management of systemic lupus erythematosus (SLE). The search was conducted on the 22nd of June 2021 for publications between 1st of January 2000 and the date of search. Additional articles within the aforementioned timeframe and until December 2023 were added by hand searching. Databases utilized were Medline, Embase, Web of Science, and Cinahl. Lifestyle interventions were defined as any intervention encompassing one or more of the following: physical exercise, diet and nutrition, mental health, harmful exposures, sleep, and social relations. The Joanna Briggs Institute critical appraisal tools were used for risk of bias assessment. The search yielded 11,274 unique records, we assessed the full text of 199 records, and finally included 102 studies. Overall, the quality of the evidence is limited, and there were multiple sources of heterogeneity. The two domains most extensively researched were mental health (40 records) and physical exercise (39 records). Psychological interventions had a positive effect on depressive symptoms, anxiety, and health-related quality of life (HRQoL), whereas physical exercise improved fatigue, depressive symptoms, aerobic capacity, and physical functioning. Studies on diet and nutrition (15 records) support that low fat intake and Mediterranean diet may be beneficial for reducing cardiovascular risk, but large interventional studies are lacking. Studies on harmful exposures (7 records) support photoprotection and use of sunscreen. While studies imply benefits regarding disease burden and drug efficacy in non-smokers and regarding HRQoL in normal-weight patients, more survey is needed on tobacco smoking and alcohol consumption, as well as weight control strategies. Studies on social relations (1 record) and sleep (no records) were sparse or non-existent. In conclusion, psychosocial interventions are viable for managing depressive symptoms, and exercise appears essential for reducing fatigue and improving aerobic capacity and physical function. Photoprotection should be recommended to all patients. Lifestyle interventions should be considered a complement, not a substitute, to pharmacotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that primarily affects women, often in the time between puberty and menopause [1]. Typical manifestations of the disease include fatigue, rashes, and arthritis, and up to 40% of patients develop renal involvement [2]. Over time, inflammation-induced organ damage and side effects from immunosuppressive therapies lead to increased morbidity and mortality [3]. During the past decades, much focus has been placed on clinical trials for pharmaceuticals. Despite considerable advances, the response rate to new biopharmaceuticals remains limited, and substantial proportions of patients still develop organ damage and experience severe pain, fatigue, and impaired quality of life [4,5,6,7]. The use of lifestyle changes as complementary interventions has not been thoroughly studied.
The field of lifestyle medicine is rather novel. Although the definition of a lifestyle intervention is not entirely clear, the American College of Lifestyle Medicine (ACLM) and the British Society of Lifestyle Medicine (BSLM) have similar approaches when outlining what constitutes a lifestyle intervention, dividing lifestyle into six areas. ACLM lists six fundamental domains: nutrition, exercise, stress, substance abuse, sleep, and relations [8]. BSLM also specifies six domains, as follows: healthy eating, physical activity, mental well-being, minimizing harmful substances, sleep, and healthy relations [9]. Hence, these six domains i.e., physical activity and exercise, diet and nutrition, mental health, harmful exposures, sleep, and social relations, are largely common for both taxonomies.
The utilization of non-pharmacological interventions is gaining interest within the European rheumatology community. In recent years, the European Alliance of Associations for Rheumatology (EULAR) has released reviews and recommendations regarding lifestyle behaviors in people with rheumatic and musculoskeletal diseases, focusing on exercise, diet, weight, alcohol, smoking, and work participation [10,11,12]. A previous systematic review exploring the efficacy of lifestyle habits in patients with SLE focused on physical exercise, tobacco smoking, and diet [13], and another focused on physical activity alone [14]. There is some evidence suggesting that lifestyle has an impact on developing SLE [15].
More recently, EULAR recommendations for the non-pharmacological management of SLE and systemic sclerosis were published [16], based on a large preceding systematic literature review [17]. Although comprehensive, this review did not explicitly separate lifestyle interventions from other modalities. Hence, specific information regarding lifestyle interventions targeting modifiable elements of health experience was diluted among the multitude of explored non-pharmacological management strategies. With the present review, driven by a distinct definition of a lifestyle intervention as described above, we aimed to distill the evidence concerning six specific modifiable lifestyle facets.
Methods
Definition of lifestyle intervention
For the purpose of this review, we defined a lifestyle intervention as any intervention that pertains to one or more of the following six lifestyle factors: physical activity and exercise, diet and nutrition, mental health, harmful exposures, sleep, and social relations, based on the components of lifestyle medicine described by ACLM and BSLM [8, 9]. For data synthesis, we grouped the included studies into these six categories.
Information sources and search strategy
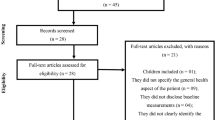
A systematic literature search was conducted on the 22nd of June 2021. Databases utilized were Medline (Ovid), Embase, Web of Science Core Collection, and CINAHL (EBSCO). We designed a broad search with two blocks, including SLE and an extensive list of non-pharmacological interventions, as a part of a larger systematic literature search [17], conducted to inform the EULAR recommendations for the non-pharmacological management of SLE and systemic sclerosis [16]. Two authors (AG, JWC) independently screened titles and abstracts of 11,251 potentially relevant records, under supervision of one senior investigator (IP). Additional articles within the aforementioned timeframe and until December 2023 were added by hand searching. The search and selection of studies were documented according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [18], as presented in Fig. 1.
Study selection
We included full reports that examined the efficacy of lifestyle interventions in patients with SLE (diagnosed according to classification criteria and/or ICD codes). Cross-sectional studies, case–control studies, cohort studies (with more than five participants), randomized control trials, and systematic reviews with meta-analyses were deemed eligible. We also included studies of mixed disease populations from which subgroup data on participants with SLE were extractable. We did not set restrictions for the duration of the interventions, or the time of outcome evaluation. We excluded records published in languages other than English, Spanish, or Swedish.
Due to the diverse nature of the design of the studies included, we did not set restrictions for the comparator group. Thus, studies were eligible if they compared groups receiving the lifestyle intervention of interest with standard of care, the lifestyle intervention with another intervention, or if they performed intra-individual comparisons i.e., before and after the intervention.
To be eligible, studies had to report data on efficacy for at least one of the following outcomes: disease activity, organ damage, health-related quality of life, functional impairment, pain, fatigue, depression and anxiety, psychological stress, or inflammatory markers.
Two authors (AG, JWC) independently screened the full text of the 176 records retrieved for eligibility and selected the records to be included, under supervision of one senior investigator (IP). Disagreements between reviewers were solved through consensus, together with two senior investigators (IP and CB).
Data collection and synthesis
Two researchers (AG and DP) independently extracted information from full texts using electronic forms, including author and year of publication, study design, size of study population, intervention and/or management strategy, comparator group, outcome measures, and key findings. These data are provided in Supplementary Table S1. Considering that we were inclusive regarding study designs and outcomes, results were summarized as reported by the authors, and comprised dichotomous and continuous data, as well as effect measures for dichotomous outcomes (risk ratios and odds ratios) and continuous outcomes (mean and standardized mean differences).
Risk of bias assessment
Risk of bias (RoB) for all included articles was conducted by one researcher (AT) using the critical appraisal (CA) checklists by the Joanna Briggs Institute [19]. Since all articles were already included before quality assessment for this review, the alternatives for overall appraisal “include”, “exclude”, and “seek further info” were modified to “robust”, “weak, and “intermediate”, respectively. The appropriate checklist for each study was selected based on study design. A study was deemed weak if there were six or more checklist items it did not clearly fulfill, intermediate if there were three to five checklist items it did not clearly fulfill, or robust if clearly fulfilling all checklist items but two or fewer. Upon RoB assessment, studies were graded by level of evidence (LoE) according to the Oxford Centre for Evidence-Based Medicine by one researcher (AT) [20].
Results
Study characteristics
After deduplication, the search yielded 11,274 unique records. We assessed the full text of 199 potentially relevant records, and finally included 102 studies. Of these, forty were categorized as studies on mental health [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60], and thirty-nine as studies on physical activity and exercise [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99]. Fifteen articles studied the effects of diet and nutrition [100,101,102,103,104,105,106,107,108,109,110,111,112,113,114], whereas seven studies examined the reduction of harmful exposures [115,116,117,118,119,120,121]. One study evaluated the effects of social relations [122], and no studies were found evaluating the effects of sleep.
Mental health
Out of forty studies examining the effects of mental health management, many focused on cognitive–behavioral therapy (CBT) [24, 26, 30,31,32, 37, 43, 48]. Two studies were meta-analyses, combining studies utilizing different psychological interventions [35, 38]. One meta-analysis found that interventions used in the constituent studies had an effect on anxiety, depressive symptoms, stress, and disease activity [35]. The other found that psychological interventions could improve depressive symptoms, and certain aspects of one’s health-related quality of life (HRQoL), but not disease activity [38]. Neither meta-analysis found any effect on fatigue [35, 38]. Three studies where randomized controlled trials (RCT) graded as LoE 2. One of these RCTs evaluated disease activity, and did not find that the intervention (biofeedback-assisted CBT) had any effect [24]. The other two RCTs showed that counseling may have a positive effect on anxiety [59] and sexual function [60], respectively. Table 1 shows the included studies of LoE 1 and 2.
Physical activity and exercise
Of the thirty-nine studies that investigated effects of physical activity and exercise, four were meta-analyses [80, 81, 86, 94]. Results showed that exercise in patients with SLE could improve HRQoL [94], decrease fatigue [80, 81], and decrease depressive symptoms [80], but not ameliorate disease activity [80]. The individual RCTs assessed as intermediate or robust in CA also found that exercise could improve fatigue [62] and depressive symptoms [76], but had no effect on disease activity [62, 76, 78] or prevention of organ damage [78]. Studies with level of evidence 1 and 2 are presented in Table 2.
The notion that physical exercise does not affect disease activity is further consolidated in other studies of non-RCT design [70, 71].
Diet and nutrition
Of the fifteen studies that investigated diet and nutrition, one was a systematic review with meta-analyses rated as intermediate in critical appraisal, whose main finding regarding SLE was that fish oil or omega-3 extracts did not affect disease activity. Two were RCTs rated as intermediate in critical appraisal. One of these suggested that turmeric extract may decrease proteinuria, hematuria, systolic blood pressure, and complement consumption [108]. However, this trial lacked between-groups comparisons in statistical analysis, so whether turmeric outperformed placebo was unclear. The other of these trials found that daily intake of green tea extract could lower disease activity after a 12-week regimen [110].
Studies with a larger sample size of non-RCT design (LoE 3; CA: robust) showed associations between (i) high intake of vitamin C [101], (ii) vitamin B6, and (iii) total dietary fiber [105] and lower disease activity, and between high vegetable fat intake and increased cardiovascular risk [101]. A cross-sectional study on the Mediterranean diet found this to be associated with both lower levels of disease activity and less organ damage accrued [112]. Table 3 shows the included studies on diet and nutrition of LoE 2 (no LoE 1 articles were found).
Harmful exposures
Of seven studies examining the relationship between SLE and harmful exposures, four examined photoprotection [115,116,117, 120]. Sunscreen has been shown to reduce expression of inflammatory markers in sun-exposed skin [117] as well as UV-radiation-induced skin lesions [115]. However, photoprotection awareness seems to have no effect on disease activity, organ damage, or lupus serology [120]. Of the other three studies, two focused on smoking [119, 121] and the other on exposure to household products [118]. Smoking was found to be associated with higher disease activity [119] and increased serum levels of B cell activating factor (BAFF) [121], but not with changes in antibody profile [119]. Use of bath salts was associated with a higher number of self-reported flare days, whereas other household products, such as adhesives and paint, were associated with less self-reported flare days, with relative risks from household product exposures ranging between 0.99 and 1.01 [118].
Social relations
One study was found evaluating social relations; this was cross-sectional in nature and found a negative association between illness uncertainty and support availability in hospitalized lupus patients [122]. From this, the authors concluded that social support should be made more accessible to these patients.
Sleep
None of the included studies examined the impact of sleeping habits.
Discussion
This systematic review examined published literature from over two decades across multiple electronic databases, aiming to summarize the evidence of lifestyle interventions for the management of systemic lupus erythematosus, a concept which has hitherto been loosely defined. Based on the emerging field of lifestyle medicine, we defined a six-domain construct to classify interventions pertaining to lifestyle. Of these six domains, mental health and physical activity and exercise were the most studied, whereas the impact of social relations and sleep appeared under-explored. Cognitive behavioral therapy seemed to reduce depressive symptoms, whereas aerobic exercise emerged as an efficacious lifestyle intervention for the amelioration of fatigue and depressive symptoms, and for the improvement of aerobic capacity.
Studies on mental health management, among which CBT was the most studied modality, showed efficacy of psychosocial interventions for improving anxiety and depressive symptoms, as well as certain aspects of HRQoL. The notion of psychological interventions reducing disease activity as reported in one meta-analysis of RCTs, although highly appealing, was not supported by the individual constituent studies used in the meta-analysis, all of them also separately included in this review, which did not show any clear effect on disease activity [24, 25, 30, 123]. Hence, this finding should be interpreted with caution.
Physical activity has a large body of evidence backing several health benefits in the general population, and a recent systematic review consolidated that aerobic training and resistance training yielded improvements in cardiovascular risk and physical function, respectively [14]. This complements the findings that physical exercise can be efficacious in managing fatigue and depressive symptoms.
Studies on diet and nutrition could be categorized into two groups: studies assessing dietary supplements, and studies assessing specific diets. The dietary supplements evaluated were broad, and included turmeric acid, green tea, and micronutrient supplements. Although some of those showed an effect on disease activity, studies included small sample sizes, had short follow-up periods, and were observational or reliant on self-reported data, precluding definite conclusions. Concerning diets, low glycemic [106], the Mediterranean [112], and the NCEP Step 2 [100] diets were explored. Among them, a study by Pocovi-Gerardino et al. assessing Mediterranean diet found improvements in body composition, cardiovascular risk, disease activity and organ damage [112]. While these findings are promising, they rely on food questionnaire data, and interventional studies evaluating the efficacy of Mediterranean diet are awaited to draw more definite conclusions.
Exposure to different elements are well-known to affect the course of SLE; naturally, sunscreen that has been shown to be efficacious [115, 117] can be complemented with other forms of photoprotection, such as wearing a hat or staying in the shade. Besides being associated with higher disease activity, smoking has been shown to reduce the efficacy of pharmacological treatment, specifically antimalarial agents [124] and belimumab [125, 126]. Another factor that is not touched upon in any of the included studies is cold exposure, which if mitigated can assist the management of Raynaud’s phenomenon [16]. Despite a sparsity of data in literature, protection from cold is now included in the EULAR recommendations for the non-pharmacological management of SLE [16]. Lastly, overweight and obesity have been associated with poor HRQoL, warranting investigation of weight loss strategies [127,128,129].
This review clarifies which lifestyle interventions have proven efficacious in relation to which outcomes, aspiring to serve as a guidance for clinicians toward implementation of such strategies. Our review also reveals the scarcity of high-level evidence regarding such interventions, and thus the need for further research, aiding researchers in the field form their research agenda.
A major drawback was that the identified studies frequently provided a poor level of evidence; there was a dearth of meta-analyses and systematic reviews that incorporated high-quality RCTs. Another concern was the substantial degree of heterogeneity across studies. This heterogeneity encompassed variations in the approach and length of interventions, evaluation instruments, comparison groups, and areas of outcomes. Nevertheless, keeping those diverse served the purpose of the broad and inclusive research questions. Some studies examined two or more interventions in parallel [66, 78], making it hard to evaluate the effect of each separate modality. Moreover, some lifestyle factors remain under-researched, and no firm conclusions can be drawn for those. Lastly, although we aligned with the lifestyle intervention categories suggested by lifestyle medicine organizations in the US and the UK, it is likely that some relevant interventions were not captured. Importantly, the identified drawbacks limit the generalizability of the results to all people living with SLE.
In conclusion, psychological interventions for mental health management and physical activity and exercise were the most studied interventions. While psychological interventions have a positive effect on depressive symptoms, anxiety, and quality of life, physical exercise improves fatigue, depressive symptoms, and physical functioning. Low-fat and Mediterranean diets constitute appealing strategies for reducing the cardiovascular risk and enhance disease control, but larger interventional studies are still required. Photoprotection remains an evidence-based recommendation for avoidance and management of cutaneous involvement. Insights regarding social relations and sleep are lacking, wherefore future studies may aim to fill this gap in knowledge. Importantly, there is an overall stronger body of evidence for medicinals for SLE [130, 131], and although several studies included in this review support efficacy of lifestyle interventions in patient-reported outcomes, hardly any evidence is provided for lifestyle interventions modulating disease activity. Hence, lifestyle interventions should be considered a complement, not a substitute, to pharmacotherapy in SLE.
Data availability
Full data relating to this review can be made available by the corresponding author upon reseanable request.
References
Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, Ruiz-Irastorza G, Hughes G (2016) Systemic lupus erythematosus. Nat Rev Dis Primers 2(1):16039. https://doi.org/10.1038/nrdp.2016.39
Hoover PJ, Costenbader KH (2016) Insights into the epidemiology and management of lupus nephritis from the US rheumatologist’s perspective. Kidney Int 90(3):487–492. https://doi.org/10.1016/j.kint.2016.03.042
Fava A, Petri M (2019) Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun 96:1–13. https://doi.org/10.1016/j.jaut.2018.11.001
Urowitz MB, Aranow C, Asukai Y, Bass DL, Bruce IN, Chauhan D, Dall’Era M, Furie R, Fox NL, Gilbride JA, Hammer A, Ginzler EM, Gonzalez-Rivera T, Levy RA, Merrill JT, Quasny H, Roth DA, Stohl W, van Vollenhoven R, Wallace DJ, Petri M (2022) Impact of belimumab on organ damage in systemic lupus erythematosus. Arthritis Care Res 74(11):1822–1828. https://doi.org/10.1002/acr.24901
Gomez A, Qiu V, Cederlund A, Borg A, Lindblom J, Emamikia S, Enman Y, Lampa J, Parodis I (2021) Adverse health-related quality of life outcome despite adequate clinical response to treatment in systemic lupus erythematosus. Front Med 8:651249. https://doi.org/10.3389/fmed.2021.651249
Gomez A, Parodis I (2022) Do biological agents improve health-related quality of life in patients with systemic lupus erythematosus? Results from a systematic search of the literature. Autoimmun Rev. https://doi.org/10.1016/j.autrev.2022.103188
Kalunian KC, Furie R, Morand EF, Bruce IN, Manzi S, Tanaka Y, Winthrop K, Hupka I, Zhang LJ, Werther S, Abreu G, Hultquist M, Tummala R, Lindholm C, Al-Mossawi H (2023) A randomized, placebo-controlled phase iii extension trial of the long-term safety and tolerability of anifrolumab in active systemic lupus erythematosus. Arthritis Rheumatol 75(2):253–265. https://doi.org/10.1002/art.42392
American College of Lifestyle Medicine (2022) What is lifestyle medicine? https://lifestylemedicine.org/ACLM/ACLM/About/What_is_Lifestyle_Medicine_/Lifestyle_Medicine.aspx?hkey=26f3eb6b-8294-4a63-83de-35d429c3bb88. Accessed 24 May 2022
British Society of Lifestyle Medicine (2022) The pillars of lifestyle medicine. https://bslm.org.uk/. Accessed 30 May 2022
Gwinnutt JM, Wieczorek M, Balanescu A, Bischoff-Ferrari HA, Boonen A, Cavalli G, de Souza S, de Thurah A, Dorner TE, Moe RH, Putrik P, Rodríguez-Carrio J, Silva-Fernández L, Stamm T, Walker-Bone K, Welling J, Zlatković-Švenda MI, Guillemin F, Verstappen SMM (2022) 2021 EULAR recommendations regarding lifestyle behaviours and work participation to prevent progression of rheumatic and musculoskeletal diseases. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2021-222020
Wieczorek M, Gwinnutt JM, Ransay-Colle M, Balanescu A, Bischoff-Ferrari H, Boonen A, Cavalli G, de Souza S, de Thurah A, Dorner TE, Moe RH, Putrik P, Rodríguez-Carrio J, Silva-Fernández L, Stamm TA, Walker-Bone K, Welling J, Zlatkovic-Svenda M, Verstappen SM, Guillemin F (2022) Smoking, alcohol consumption and disease-specific outcomes in rheumatic and musculoskeletal diseases (RMDs): systematic reviews informing the 2021 EULAR recommendations for lifestyle improvements in people with RMDs. RMD Open. https://doi.org/10.1136/rmdopen-2021-002170
Rausch Osthoff AK, Niedermann K, Braun J, Adams J, Brodin N, Dagfinrud H, Duruoz T, Esbensen BA, Günther KP, Hurkmans E, Juhl CB, Kennedy N, Kiltz U, Knittle K, Nurmohamed M, Pais S, Severijns G, Swinnen TW, Pitsillidou IA, Warburton L, Yankov Z, Vliet Vlieland TPM (2018) 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis 77(9):1251–1260. https://doi.org/10.1136/annrheumdis-2018-213585
Rodríguez Huerta MD, Trujillo-Martín MM, Rúa-Figueroa Í, Cuellar-Pompa L, Quirós-López R, Serrano-Aguilar P, Spanish SLECPGDG (2016) Healthy lifestyle habits for patients with systemic lupus erythematosus: a systemic review. Semin Arthritis Rheum 45(4):463–470. https://doi.org/10.1016/j.semarthrit.2015.09.003
Blaess J, Goepfert T, Geneton S, Irenee E, Gerard H, Taesch F, Sordet C, Arnaud L (2023) Benefits & risks of physical activity in patients with Systemic Lupus Erythematosus: a systematic review of the literature. Semin Arthritis Rheum 58:152128. https://doi.org/10.1016/j.semarthrit.2022.152128
Choi MY, Hahn J, Malspeis S, Stevens EF, Karlson EW, Sparks JA, Yoshida K, Kubzansky L, Costenbader KH (2022) Association of a combination of healthy lifestyle behaviors with reduced risk of incident systemic lupus erythematosus. Arthritis Rheumatol 74(2):274–283. https://doi.org/10.1002/art.41935
Parodis I, Girard-Guyonvarc’h C, Arnaud L, Distler O, Domján A, Van den Ende CHM, Fligelstone K, Kocher A, Larosa M, Lau M, Mitropoulos A, Ndosi M, Poole JL, Redmond A, Ritschl V, Alexanderson H, Sjöberg Y, von Perner G, Uhlig T, Varju C, Vriezekolk JE, Welin E, Westhovens R, Stamm TA, Boström C (2023) EULAR recommendations for the non-pharmacological management of systemic lupus erythematosus and systemic sclerosis. Ann Rheum Dis. https://doi.org/10.1136/ard-2023-224416
Parodis I, Gomez A, Tsoi A, Chow JW, Pezzella D, Girard C, Stamm TA, Boström C (2023) Systematic literature review informing the EULAR recommendations for the non-pharmacological management of systemic lupus erythematosus and systemic sclerosis. RMD Open. https://doi.org/10.1136/rmdopen-2023-003297
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Aromataris E, Munn Z (eds) (2020) JBI manual for evidence synthesis. https://synthesismanual.jbi.global. Accessed 25 Feb 25 2022
Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati, Alessandro, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M (2011) The oxford levels of evidence 2. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed 5 Mar 2024
Sohng KY (2003) Effects of a self-management course for patients with systemic lupus erythematosus. J Adv Nurs 42(5):479–486
Brown SJ, Somerset ME, McCabe CS, McHugh NJ (2004) The impact of group education on participants’ management of their disease in lupus and scleroderma. Musculoskeletal Care 2(4):207–217
Dorsey RR, Andresen EM, Moore TL (2004) Health-related quality of life and support group attendance for patients with systemic lupus erythematosus. JCR J Clin Rheumatol 10(1):6–9
Greco CM, Rudy TE, Manzi S (2004) Effects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: a randomized controlled trial. Arthritis Rheum 51(4):625–634
Karlson EW, Liang MH, Eaton H, Huang J, Fitzgerald L, Rogers MP, Daltroy LH (2004) A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum 50(6):1832–1841
Goodman D, Morrissey S, Graham D, Bossingham D (2005) The application of cognitive-behaviour therapy in altering illness representations of systemic lupus erythematosus. Behav Chang 22(3):156–171
Harrison MJ, Morris KA, Horton R, Toglia J, Barsky J, Chait S, Ravdin L, Robbins L (2005) Results of intervention for lupus patients with self-perceived cognitive difficulties. Neurology 65(8):1325–1327
Haupt M, Millen S, Janner M, Falagan D, Fischer-Betz R, Schneider M (2005) Improvement of coping abilities in patients with systemic lupus erythematosus: a prospective study. Ann Rheum Dis 64(11):1618–1623
Miljeteig K, Graue M (2009) Evaluation of a multidisciplinary patient education program for people with systemic lupus erythematosus. J Nurs Healthc Chronic Illnesses 1(1):87–95
Navarrete-Navarrete N, Peralta-Ramirez MI, Sabio-Sanchez JM, Coin MA, Robles-Ortega H, Hidalgo-Tenorio C, Ortego-Centeno N, Callejas-Rubio JL, Jimenez-Alonso J (2010) Efficacy of cognitive behavioural therapy for the treatment of chronic stress in patients with lupus erythematosus: a randomized controlled trial. Psychother Psychosom 79(2):107–115
Navarrete-Navarrete N, Peralta-Ramirez MI, Sabio JM, Martinez-Egea I, Santos-Ruiz A, Jimenez-Alonso J (2010) Quality-of-life predictor factors in patients with SLE and their modification after cognitive behavioural therapy. Lupus 19(14):1632–1639
Brown RT, Shaftman SR, Tilley BC, Anthony KK, Kral MC, Maxson B, Mee L, Bonner MJ, Vogler LB, Schanberg LE, Connelly MA, Wagner JL, Silver RM, Nietert PJ (2012) The health education for lupus study: a randomized controlled cognitive-behavioral intervention targeting psychosocial adjustment and quality of life in adolescent females with systemic lupus erythematosus. Am J Med Sci 344(4):274–282
Drenkard C, Dunlop-Thomas C, Easley K, Bao G, Brady T, Lim SS (2012) Benefits of a self-management program in low-income African–American women with systemic lupus erythematosus: results of a pilot test. Lupus 21(14):1586–1593
Ganachari MS, Almas SA (2012) Evaluation of clinical pharmacist mediated education and counselling of systemic lupus erythematosus patients in tertiary care hospital. Indian J Rheumatol 7(1):7–12
Zhang J, Wei W, Wang CM (2012) Effects of psychological interventions for patients with systemic lupus erythematosus: a systematic review and meta-analysis. Lupus 21(10):1077–1087
Bantornwan S, Watanapa WB, Hussarin P, Chatsiricharoenkul S, Larpparisuth N, Teerapornlertratt T, Vareesangthip J, Vareesangthip K (2014) Role of meditation in reducing sympathetic hyperactivity and improving quality of life in lupus nephritis patients with chronic kidney disease. J Med Assoc Thai 97:S101-107
Jolly M, Peters KF, Mikolaitis R, Evans-Raoul K, Block JA (2014) Body image intervention to improve health outcomes in lupus: a pilot study. JCR J Clin Rheumatol 20(8):403–410
Liang H, Tian X, Cao LY, Chen YY, Wang CM (2014) Effect of psychological intervention on healthrelated quality of life in people with systemic lupus erythematosus: a systematic review. Int J Nurs Sci 1(3):298–305
Williams EM, Penfield M, Kamen D, Oates JC (2014) An intervention to reduce psychosocial and biological indicators of stress in African American lupus patients: the balancing lupus experiences with stress strategies study. Open J Prev Med 4(1):22–31. https://doi.org/10.4236/ojpm.2014.41005
Williams EM, Bruner L, Penfield M, Kamen D, Oates JC (2014) Stress and depression in relation to functional health behaviors in African American patients with systemic lupus erythematosus. Rheumatology. https://doi.org/10.4172/2161-1149.S4-005
Horesh D, Glick I, Taub R, Agmon-Levin N, Shoenfeld Y (2017) Mindfulness-based group therapy for systemic lupus erythematosus: a first exploration of a promising mind-body intervention. Complement Ther Clin Pract 26:73–75
O’Riordan R, Doran M, Connolly D (2017) Fatigue and activity management education for individuals with systemic lupus erythematosus. Occup Ther Int 2017:4530104
Solati K, Mousavi M, Kheiri S, Hasanpour-Dehkordi A (2017) The effectiveness of mindfulness-based cognitive therapy on psychological symptoms and quality of life in systemic lupus erythematosus patients: a randomized controlled trial. Oman Med J 32(5):378–385
Yelnik CM, Richey M, Haiduc V, Everett S, Zhang M, Erkan D (2017) Cardiovascular disease prevention counseling program for systemic lupus erythematosus patients. Arthritis Care Res 69(8):1209–1216
Kusnanto K, Sari N, Harmayetty H, Efendi F, Gunawan J (2018) Self-care model application to improve self-care agency, self-care activities, and quality of life in patients with systemic lupus erythematosus. J Taibah Univ Med Sci 13(5):472–478
Scalzi LV, Hollenbeak CS, Mascuilli E, Olsen N (2018) Improvement of medication adherence in adolescents and young adults with SLE using web-based education with and without a social media intervention, a pilot study. Pediatr Rheumatol 16(1):18
Williams EM, Hyer JM, Viswanathan R, Faith TD, Voronca D, Gebregziabher M, Oates JC, Egede L (2018) Peer-to-peer mentoring for African American women with lupus: a feasibility pilot. Arthritis Care Res 70(6):908–917
Kim HA, Seo L, Jung JY, Kim YW, Lee E, Cho SM, Suh CH (2019) Mindfulness-based cognitive therapy in Korean patients with systemic lupus erythematosus: a pilot study. Complement Ther Clin Pract 35:18–21
Sahebari M, Asghari Ebrahimabad MJ, Ahmadi Shoraketokanlo A, Aghamohammadian Sharbaf H, Khodashahi M (2019) Efficacy of acceptance and commitment therapy in reducing disappointment, psychological distress, and psychasthenia among systemic lupus erythematosus (SLE) patients. Iran J Psychiatry 14(2):130–136
Williams EM, Dismuke CL, Faith TD, Smalls BL, Brown E, Oates JC, Egede LE (2019) Cost-effectiveness of a peer mentoring intervention to improve disease self-management practices and self-efficacy among African American women with systemic lupus erythematosus: analysis of the Peer Approaches to Lupus Self-management (PALS) pilot study. Lupus 28(8):937–944
Kankaya H, Karadakovan A (2020) Effects of web-based education and counselling for patients with systemic lupus erythematosus: self-efficacy, fatigue and assessment of care. Lupus 29(8):884–891. https://doi.org/10.1177/0961203320928423
Khan F, Granville N, Malkani R, Chathampally Y (2020) Health-related quality of life improvements in systemic lupus erythematosus derived from a digital therapeutic plus tele-health coaching intervention: randomized controlled pilot trial. J Med Internet Res 22(10):e23868. https://doi.org/10.2196/23868
Allen KD, Beauchamp T, Rini C, Keefe FJ, Bennell KL, Cleveland RJ, Grimm K, Huffman K, Hu DG, Santana A, Saxena Beem S, Walker J, Sheikh SZ (2021) Pilot study of an internet-based pain coping skills training program for patients with systemic Lupus Erythematosus. BMC Rheumatol 5(1):20. https://doi.org/10.1186/s41927-021-00191-6
White AA, Ba A, Faith TD, Ramakrishnan V, Dismuke-Greer CL, Oates JC, Williams EM (2021) The Care-coordination Approach to Learning Lupus Self-Management: a patient navigator intervention for systemic lupus inpatients. Lupus Sci Med 8(1):e000482
Xu HY, Teng Q, Zeng Y, Tian CP, Yang BW, Yao XL (2021) Psychoeducational intervention benefits the quality of life of patients with active systemic lupus erythematosus. J Nanomater. https://doi.org/10.1155/2021/9967676
McCormick EM, Englund TR, Cleveland RJ, Dickson TA, Schiller CE, Sheikh SZ (2022) ACT for lupus: pilot feasibility and acceptability study of a novel web-based acceptance and commitment therapy program for patients with lupus. ACR Open Rheumatol 4(7):574–580. https://doi.org/10.1002/acr2.11433
Kang J, Zhu X, Kan Y, Zhuang S (2023) Application of the knowledge, attitude, and practice model combined with motivational interviewing for health education in female patients with systemic lupus erythematosus. Medicine 102(12):e33338. https://doi.org/10.1097/md.0000000000033338
Kawka L, Sarmiento-Monroy JC, Mertz P, Pijnenburg L, Rinagel M, Ugarte-Gil MF, Geneton S, Blaess J, Piga M, Arnaud L (2023) Assessment and personalised advice for fatigue in systemic lupus erythematosus using an innovative digital tool: the Lupus Expert system for the Assessment of Fatigue (LEAF) study. RMD Open. https://doi.org/10.1136/rmdopen-2023-003476
Pasyar N, Sam A, Rivaz M, Nazarinia M (2023) A smartphone-based supportive counseling on health anxiety and acceptance of disability in Systemic Lupus Erythematosus patients: a randomized clinical trial. Patient Educ Couns 110:107676. https://doi.org/10.1016/j.pec.2023.107676
Shami M, Montazeri A, Faezi ST, Behboodi Moghadam Z (2023) The effect of sexual counseling based on EX-PLISSIT model on improving the sexual function of married women with systemic lupus erythematosus: a randomized controlled trial. Sex Disabil 41(2):451–466. https://doi.org/10.1007/s11195-023-09776-0
Ramsey-Goldman R, Schilling EM, Dunlop D, Langman C, Greenland P, Thomas RJ, Chang RW (2000) A pilot study on the effects of exercise in patients with systemic lupus erythematosus. Arthritis Care Res 13(5):262–269
Tench CM, McCarthy J, McCurdie I, White PD, D’Cruz DP (2003) Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology 42(9):1050–1054
Carvalho MR, Sato EI, Tebexreni AS, Heidecher RT, Schenkman S, Neto TL (2005) Effects of supervised cardiovascular training program on exercise tolerance, aerobic capacity, and quality of life in patients with systemic lupus erythematosus. Arthritis Rheum 53(6):838–844
Clarke-Jenssen AC, Fredriksen PM, Lilleby V, Mengshoel AM (2005) Effects of supervised aerobic exercise in patients with systemic lupus erythematosus: a pilot study. Arthritis Rheum 53(2):308–312
do Prado DM, Gualano B, Miossi R, Lima FR, Roschel H, Borba E, Bonfa E, de Sa Pinto AL (2011) Erratic control of breathing during exercise in patients with systemic lupus erythematosus: a pilot-study. Lupus 20(14):1535–1540
Otto AD, Mishler AE, Shah N, Krug MM, Phillips A, Wilson N, Kao AH (2011) Feasibility of implementing a lifestyle intervention in overweight and obese patients with systemic lupus erythematosus. Med Sci Sports Exerc 43:123–123
Yuen HK, Holthaus K, Kamen DL, Sword DO, Breland HL (2011) Using Wii Fit to reduce fatigue among African American women with systemic lupus erythematosus: a pilot study. Lupus 20(12):1293–1299
Miossi R, Benatti FB, de Sa L, Pinto A, Lima FR, Borba EF, Prado DM, Perandini LA, Gualano B, Bonfa E, Roschel H (2012) Using exercise training to counterbalance chronotropic incompetence and delayed heart rate recovery in systemic lupus erythematosus: a randomized trial. Arthritis Care Res 64(8):1159–1166
da Silva AE, dos Reis-Neto ET, da Silva NP, Sato EI (2013) The effect of acute physical exercise on cytokine levels in patients with systemic lupus erythematosus. Lupus 22(14):1479–1483
dos Reis-Neto ET, da Silva AE, Monteiro CM, de Camargo LM, Sato EI (2013) Supervised physical exercise improves endothelial function in patients with systemic lupus erythematosus. Rheumatology 52(12):2187–2195
Barnes JN, Nualnim N, Dhindsa M, Renzi CP, Tanaka H (2014) Macro- and microvascular function in habitually exercising systemic lupus erythematosus patients. Scand J Rheumatol 43(3):209–216
Perandini LA, Sales-de-Oliveira D, Mello SB, Camara NO, Benatti FB, Lima FR, Borba E, Bonfa E, Sa-Pinto AL, Roschel H, Gualano B (2014) Exercise training can attenuate the inflammatory milieu in women with systemic lupus erythematosus. J Appl Physiol 117(6):639–647
Benatti FB, Miossi R, Passareli M, Nakandakare ER, Perandini L, Lima FR, Roschel H, Borba E, Bonfa E, Gualano B, de Sa Pinto AL (2015) The effects of exercise on lipid profile in systemic lupus erythematosus and healthy individuals: a randomized trial. Rheumatol Int 35(1):61–69
Bogdanovic G, Stojanovich L, Djokovic A, Stanisavljevic N (2015) Physical activity program is helpful for improving quality of life in patients with systemic lupus erythematosus. Tohoku J Exp Med 237(3):193–199
Perandini LA, Sales-de-Oliveira D, Mello S, Camara NO, Benatti FB, Lima FR, Borba E, Bonfa E, Roschel H, Sa-Pinto AL, Gualano B (2015) Inflammatory cytokine kinetics to single bouts of acute moderate and intense aerobic exercise in women with active and inactive systemic lupus erythematosus. Exerc Immunol Rev 21:174–185
Abrahao MI, Gomiero AB, Peccin MS, Grande AJ, Trevisani VF (2016) Cardiovascular training vs. resistance training for improving quality of life and physical function in patients with systemic lupus erythematosus: a randomized controlled trial. Scand J Rheumatol 45(3):197–201
Avaux M, Hoellinger P, Nieuwland-Husson S, Fraselle V, Depresseux G, Houssiau FA (2016) Effects of two different exercise programs on chronic fatigue in lupus patients. Acta Clin Belg 71(6):403–406
Bostrom C, Elfving B, Dupre B, Opava CH, Lundberg IE, Jansson E (2016) Effects of a one-year physical activity programme for women with systemic lupus erythematosus—a randomized controlled study. Lupus 25(6):602–616
Perandini LA, Sales-de-Oliveira D, Almeida DC, Azevedo H, Moreira-Filho CA, Cenedeze MA, Benatti FB, Lima FR, Borba E, Bonfa E, Sa-Pinto AL, Roschel H, Camara NO, Gualano B (2016) Effects of acute aerobic exercise on leukocyte inflammatory gene expression in systemic lupus erythematosus. Exerc Immunol Rev 22:64–81
O’Dwyer T, Durcan L, Wilson F (2017) Exercise and physical activity in systemic lupus erythematosus: a systematic review with meta-analyses. Semin Arthritis Rheum 47(2):204–215
Wu ML, Yu KH, Tsai JC (2017) The effectiveness of exercise in adults with systemic lupus erythematosus: a systematic review and meta-analysis to guide evidence-based practice. Worldview Evid-Based Nurs 14(4):306–315
Benatti FB, Miyake CNH, Dantas WS, Zambelli VO, Shinjo SK, Pereira RMR, Silva MER, Sa-Pinto AL, Borba E, Bonfa E, Gualano B (2018) Exercise increases insulin sensitivity and skeletal muscle AMPK expression in systemic lupus erythematosus: a randomized controlled trial. Front Immunol 9:906
Middleton KR, Haaz Moonaz S, Hasni SA, Magana Lopez M, Tataw-Ayuketah G, Farmer N, Wallen GR (2018) Yoga for systemic lupus erythematosus (SLE): clinician experiences and qualitative perspectives from students and yoga instructors living with SLE. Complement Ther Med 41:111–117
Soriano-Maldonado A, Morillas-de-Laguno P, Sabio JM, Gavilan-Carrera B, Rosales-Castillo A, Montalban-Mendez C, Saez-Uran LM, Callejas-Rubio JL, Vargas-Hitos JA (2018) Effects of 12-week aerobic exercise on arterial stiffness, inflammation, and cardiorespiratory fitness in women with systemic LUPUS erythematosus: non-randomized controlled trial. J Clin Med 7(12):24
Timoteo RP, Silva AF, Micheli DC, Candido Murta EF, Freire M, Teodoro RB, Lima FM, Martins Tavares Murta B, Bertoncello D (2018) Increased flexibility, pain reduction and unaltered levels of IL-10 and CD11b + lymphocytes in patients with systemic lupus erythematosus were associated with kinesiotherapy. Lupus 27(7):1159–1168
da Hora TC, Lima K, Maciel R (2019) The effect of therapies on the quality of life of patients with systemic lupus erythematosus: a meta-analysis of randomized trials. Adv Rheumatol 59(1):34
Sheikh SZ, Kaufman K, Gordon BB, Hicks S, Love A, Walker J, Callahan LF, Cleveland RJ (2019) Evaluation of the self-directed format of Walk With Ease in patients with systemic lupus erythematosus: the Walk-SLE Pilot Study. Lupus 28(6):764–770
Wu ML, Tsai JC, Yu KH, Chen JJ (2019) Effects of physical activity counselling in women with systemic lupus erythematosus: a randomized controlled trial. Int J Nurs Pract 25(5):e12770
Gavilan-Carrera B, Vargas-Hitos JA, Morillas-de-Laguno P, Rosales-Castillo A, Sola-Rodriguez S, Callejas-Rubio JL, Sabio JM, Soriano-Maldonado A (2020) Effects of 12-week aerobic exercise on patient-reported outcomes in women with systemic lupus erythematosus. Disabil Rehabil. https://doi.org/10.1080/09638288.2020.1808904
Keramiotou K, Anagnostou C, Kataxaki E, Galanos A, Sfikakis PP, Tektonidou MG (2020) The impact of upper limb exercise on function, daily activities and quality of life in systemic lupus erythematosus: a pilot randomised controlled trial. RMD Open 6(1):01
Dionello CF, Souza PL, Rosa PV, Santana A, Marchon R, Morel DS, Moreira-Marconi E, Frederico EFF, Sa-Caputo DC, Coelho-Oliveira AC, Crivelli M, Klumb EM, Taiar R, Marin PJ, Bernardo M (2021) Acute neuromuscular responses to whole-body vibration of systemic lupus erythematosus individuals: a randomized pilot study. Appl Sci-Basel 11(1):138
Kao VP, Wen HJ, Pan YJ, Pai CS, Tsai ST, Su KY (2021) Combined aerobic and resistance training improves physical and executive functions in women with systemic lupus erythematosus. Lupus 30(6):946–955
Lopes-Souza P, Dionello CF, Bernardes-Oliveira CL, Moreira-Marconi E, Marchon RM, Teixeira-Silva Y, Paineiras-Domingos LL, da Cunha S-C, Xavier VL, Bergmann A, Klumb EM, Bernardo-Filho M (2021) Effects of 12-week whole-body vibration exercise on fatigue, functional ability and quality of life in women with systemic lupus erythematosus: a randomized controlled trial. J Bodyw Mov Ther 27:191–199
Lu MC, Koo M (2021) Effects of exercise intervention on health-related quality of life in patients with systemic lupus erythematosus: a systematic review and meta-analysis of controlled trials. Healthcare. https://doi.org/10.3390/healthcare9091215
Patterson SL, Trupin L, Yazdany J, Dall’Era M, Lanata C, Dequattro K, Hartogensis W, Katz P (2021) Physical inactivity independently predicts incident depression in a multi-racial/ethnic systemic lupus cohort. Arthritis Care Res 9:09
Hashemi S, Habibagahi Z, Heidari M, Abdollahpour-Alitappeh M, Karimi MH (2022) Effects of combined aerobic and anaerobic exercise training on cytokine profiles in patients with systemic lupus erythematosus (SLE); a randomized controlled trial. Transpl Immunol 70:101516. https://doi.org/10.1016/j.trim.2021.101516
Frade S, O’Neill S, Walsh S, Campbell C, Greene D, Bird SP, Cameron M (2023) Telehealth-supervised exercise in systemic lupus erythematosus: a pilot study. Lupus 32(4):508–520. https://doi.org/10.1177/09612033231157073
Lin MC, Livneh H, Lu MC, Chang CH, Chen ML, Tsai TY (2023) Effects of a walking exercise programme on disease activity, sleep quality, and quality of life in systemic lupus erythematosus patients. Int J Nurs Pract 29(6):e13174. https://doi.org/10.1111/ijn.13174
Wohland H, Aringer M, Leuchten N (2023) Physical exercise is associated with less fatigue, less pain and better sleep in patients with systemic lupus erythematosus. Clin Exp Rheumatol. https://doi.org/10.55563/clinexprheumatol/az3pkn
Shah M, Kavanaugh A, Coyle Y, Adams-Huet B, Lipsky PE (2002) Effect of a culturally sensitive cholesterol lowering diet program on lipid and lipoproteins, body weight, nutrient intakes, and quality of life in patients with systemic lupus erythematosus. J Rheumatol 29(10):2122–2128
Minami Y, Sasaki T, Arai Y, Kurisu Y, Hisamichi S (2003) Diet and systemic lupus erythematosus: a 4 year prospective study of Japanese patients. J Rheumatol 30(4):747–754
Duffy EM, Meenagh GK, McMillan SA, Strain JJ, Hannigan BM, Bell AL (2004) The clinical effect of dietary supplementation with omega-3 fish oils and/or copper in systemic lupus erythematosus. J Rheumatol 31(8):1551–1556
Shah M, Adams-Huet B, Kavanaugh A, Coyle Y, Lipsky P (2004) Nutrient intake and diet quality in patients with systemic lupus erythematosus on a culturally sensitive cholesterol lowering dietary program. J Rheumatol 31(1):71–75
Aghdassi E, Morrison S, Landolt-Marticorena C, Su J, Pineau CA, Gladman D, Urowitz M, Pope J, Peschken C, Ca NL, Investigators H, Dacosta D, Wither J, Fortin PR (2010) The use of micronutrient supplements is not associated with better quality of life and disease activity in Canadian patients with systemic lupus erythematosus. J Rheumatol 37(1):87–90. https://doi.org/10.3899/jrheum.090761
Minami Y, Hirabayashi Y, Nagata C, Ishii T, Harigae H, Sasaki T (2011) Intakes of vitamin B6 and dietary fiber and clinical course of systemic lupus erythematosus: a prospective study of Japanese female patients. J Epidemiol 21(4):246–254
Davies RJ, Lomer MC, Yeo SI, Avloniti K, Sangle SR, D’Cruz DP (2012) Weight loss and improvements in fatigue in systemic lupus erythematosus: a controlled trial of a low glycaemic index diet versus a calorie restricted diet in patients treated with corticosteroids. Lupus 21(6):649–655
Elkan AC, Anania C, Gustafsson T, Jogestrand T, Hafstrom I, Frostegard J (2012) Diet and fatty acid pattern among patients with SLE: associations with disease activity, blood lipids and atherosclerosis. Lupus 21(13):1405–1411
Khajehdehi P, Zanjaninejad B, Aflaki E, Nazarinia M, Azad F, Malekmakan L, Dehghanzadeh GR (2012) Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study. J Ren Nutr 22(1):50–57
Everett ST, Wolf R, Contento I, Haiduc V, Richey M, Erkan D (2015) Short-term patient-centered nutrition counseling impacts weight and nutrient intake in patients with systemic lupus erythematosus. Lupus 24(12):1321–1326
Shamekhi Z, Amani R, Habibagahi Z, Namjoyan F, Ghadiri A, Saki Malehi A (2017) A randomized, double-blind, placebo-controlled clinical trial examining the effects of green tea extract on systemic lupus erythematosus disease activity and quality of life. Phytother Res 31(7):1063–1071
Rothman D, Khan F, Rudin V (2018) Individualized diet and lifestyle modifications reverse symptoms of systemic lupus erythematosus. J Med Internet Res 20(9):21–21
Pocovi-Gerardino G, Correa-Rodriguez M, Callejas-Rubio JL, Rios-Fernandez R, Martin-Amada M, Cruz-Caparros MG, Rueda-Medina B, Ortego-Centeno N (2021) Beneficial effect of Mediterranean diet on disease activity and cardiovascular risk in systemic lupus erythematosus patients: a cross-sectional study. Rheumatology 60(1):160–169
Gwinnutt JM, Wieczorek M, Rodríguez-Carrio J, Balanescu A, Bischoff-Ferrari HA, Boonen A, Cavalli G, de Souza S, de Thurah A, Dorner TE, Moe RH, Putrik P, Silva-Fernández L, Stamm T, Walker-Bone K, Welling J, Zlatković-Švenda M, Guillemin F, Verstappen SMM (2022) Effects of diet on the outcomes of rheumatic and musculoskeletal diseases (RMDs): systematic review and meta-analyses informing the 2021 EULAR recommendations for lifestyle improvements in people with RMDs. RMD Open. https://doi.org/10.1136/rmdopen-2021-002167
Knippenberg A, Robinson GA, Wincup C, Ciurtin C, Jury EC, Kalea AZ (2022) Plant-based dietary changes may improve symptoms in patients with systemic lupus erythematosus. Lupus 31(1):65–76. https://doi.org/10.1177/09612033211063795
Stege H, Budde MA, Grether-Beck S, Krutmann J (2000) Evaluation of the capacity of sunscreens to photoprotect lupus erythematosus patients by employing the photoprovocation test. Photodermatol Photoimmunol Photomed 16(6):256–259
Herzinger T, Plewig G, Rocken M (2004) Use of sunscreens to protect against ultraviolet-induced lupus erythematosus. Arthritis Rheum 50(9):3045–3046
Zahn S, Graef M, Patsinakidis N, Landmann A, Surber C, Wenzel J, Kuhn A (2014) Ultraviolet light protection by a sunscreen prevents interferon-driven skin inflammation in cutaneous lupus erythematosus. Exp Dermatol 23(7):516–518
Squance ML, Reeves G, Attia J, Bridgman H, Guest M (2015) Self-reported Lupus flare: association with everyday home and personal product exposure. Toxicol Rep 2:880–888
Xu D, You X, Wang Z, Zeng Q, Xu J, Jiang L, Gong L, Wu F, Gu J, Tao Y, Chen J, Zhao J, Li M, Zhao Y, Zeng X, CSTAR co-authors (2015) Chinese systemic lupus erythematosus treatment and research group registry VI: effect of cigarette smoking on the clinical phenotype of Chinese patients with systemic lupus erythematosus. PLoS ONE 10(8):e0134451
Abdul Kadir WD, Jamil A, Shaharir SS, Md Nor N, Abdul Gafor AH (2018) Photoprotection awareness and practices among patients with systemic lupus erythematosus and its association with disease activity and severity. Lupus 27(8):1287–1295
Raymond WD, Hamdorf M, Furfaro M, Eilertsen GO, Nossent JC (2021) Smoking associates with increased BAFF and decreased interferon-γ levels in patients with systemic lupus erythematosus. Lupus Sci Med. https://doi.org/10.1136/lupus-2021-000537
Li X, He L, Wang J, Wang M (2019) Illness uncertainty, social support, and coping mode in hospitalized patients with systemic lupus erythematosus in a hospital in Shaanxi, China. PLoS ONE 14(2):e0211313
Dobkin PL, Da Costa D, Joseph L, Fortin PR, Edworthy S, Barr S, Ensworth S, Esdaile JM, Beaulieu A, Zummer M, Senecal JL, Goulet JR, Choquette D, Rich E, Smith D, Cividino A, Gladman D, St-Pierre Y, Clarke AE (2002) Counterbalancing patient demands with evidence: results from a pan-Canadian randomized clinical trial of brief supportive-expressive group psychotherapy for women with systemic lupus erythematosus. Ann Behav Med 24(2):88–99
Parisis D, Bernier C, Chasset F, Arnaud L (2019) Impact of tobacco smoking upon disease risk, activity and therapeutic response in systemic lupus erythematosus: a systematic review and meta-analysis. Autoimmun Rev 18(11):102393. https://doi.org/10.1016/j.autrev.2019.102393
Parodis I, Sjöwall C, Jönsen A, Ramsköld D, Zickert A, Frodlund M, Sohrabian A, Arnaud L, Rönnelid J, Malmström V, Bengtsson AA, Gunnarsson I (2017) Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev 16(4):343–351. https://doi.org/10.1016/j.autrev.2017.02.005
Parodis I, Gomez A, Frodlund M, Jönsen A, Zickert A, Sjöwall C, Bengtsson AA, Gunnarsson I (2018) Smoking reduces the efficacy of belimumab in mucocutaneous lupus. Expert Opin Biol Ther 18(8):911–920. https://doi.org/10.1080/14712598.2018.1494719
Borg A, Gomez A, Cederlund A, Cobar F, Qiu V, Lindblom J, Emamikia S, Enman Y, Pettersson S, Parodis I (2021) Contribution of abnormal BMI to adverse health-related quality of life outcomes after a 52-week therapy in patients with SLE. Rheumatology 60(9):4205–4217. https://doi.org/10.1093/rheumatology/keaa909
Gomez A, Hani Butrus F, Johansson P, Åkerström E, Soukka S, Emamikia S, Enman Y, Pettersson S, Parodis I (2021) Impact of overweight and obesity on patient-reported health-related quality of life in systemic lupus erythematosus. Rheumatology 60(3):1260–1272. https://doi.org/10.1093/rheumatology/keaa453
Borg A, Lindblom J, Gomez A, Soltani A, Enman Y, Heintz E, Regardt M, Grannas D, Emamikia S, Parodis I (2023) Obesity is associated with pain and impaired mobility despite therapy in systemic lupus erythematosus. Front Med. https://doi.org/10.3389/fmed.2023.1247354
Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, Cervera R, Doria A, Gordon C, Govoni M, Houssiau F, Jayne D, Kouloumas M, Kuhn A, Larsen JL, Lerstrøm K, Moroni G, Mosca M, Schneider M, Smolen JS, Svenungsson E, Tesar V, Tincani A, Troldborg A, van Vollenhoven R, Wenzel J, Bertsias G, Boumpas DT (2019) 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 78(6):736–745. https://doi.org/10.1136/annrheumdis-2019-215089
Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, Boletis J, Frangou E, Houssiau FA, Hollis J, Karras A, Marchiori F, Marks SD, Moroni G, Mosca M, Parodis I, Praga M, Schneider M, Smolen JS, Tesar V, Trachana M, van Vollenhoven RF, Voskuyl AE, Teng YKO, van Leew B, Bertsias G, Jayne D, Boumpas DT (2020) 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 79(6):713–723. https://doi.org/10.1136/annrheumdis-2020-216924
Acknowledgements
The authors thank Emma-Lotta Säätelä, librarian at Karolinska Institutet, for aiding the literature search.
Funding
Open access funding provided by Karolinska Institute. IP has received grants from the Swedish Rheumatism Association (R-969696), King Gustaf V’s 80-year Foundation (FAI-2020-0741), Swedish Society of Medicine (SLS-974449), Nyckelfonden (OLL-974804), Professor Nanna Svartz Foundation (2021-00436), Ulla and Roland Gustafsson Foundation (2021-26), Region Stockholm (FoUI-955483), and Karolinska Institutet. CB has received grants from the Swedish Rheumatism Association and Norrbacka-Eugenia stiftelsen.
Author information
Authors and Affiliations
Contributions
Study conception and design: CB, IP. Literature search and data extraction: AT, AG, DP, JWC. Supervision: CB, CGG, TS, LA, IP. Manuscript writing – initial draft: AT and IP. Manuscript writing – critical review and revisions: all authors. All authors have approved the final version of the manuscript and take full responsibility for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
IP has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia, Bristol-Myers Squibb, Elli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Otsuka, and Roche. All other authors declare that they have no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsoi, A., Gomez, A., Boström, C. et al. Efficacy of lifestyle interventions in the management of systemic lupus erythematosus: a systematic review of the literature. Rheumatol Int 44, 765–778 (2024). https://doi.org/10.1007/s00296-024-05548-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-024-05548-x