Abstract
To establish practical recommendations for the management of patients with psoriatic arthritis (PsA) with particular clinical situations that might lead to doubts in the pharmacological decision-making. A group of six expert rheumatologists on PsA identified particular clinical situations in PsA. Then, a systematic literature review (SLR) was performed to analyse the efficacy and safety of csDMARDs, b/tsDMARDs in PsA. In a nominal group meeting, the results of the SLR were discussed and a set of recommendations were proposed for a Delphi process. A total of 65 rheumatologists were invited to participate in the Delphi. Agreement was defined if ≥ 70% of the participants voted ≥ 7 (from 1, totally disagree to 10, totally agree). For each recommendation, the level of evidence and grade of recommendation was established based on the Oxford Evidence-Based Medicine categorisation. Particular clinical situations included monoarthritis, axial disease, or non-musculoskeletal manifestations. The SLR finally comprised 131 articles. A total of 16 recommendations were generated, all but 1 reached consensus. According to them, it is crucial to carefully analyse the impact of individual manifestations on patients (disability, quality of life, etc.), but also to recognise the impact of each drug singularities on selected clinical phenotypes to adopt the most appropriate treatment strategy. Early diagnosis and treatment to target approach, along with a close risk management, is also necessary. These recommendations are intended to complement gaps in national and international guidelines by helping health professionals address and manage particular clinical situations in PsA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriatic arthritis (PsA) is a heterogeneous, potentially severe and complex disease [1]. It comprises multiple disease manifestations, including peripheral arthritis, enthesitis, dactylitis, spondylitis, skin and nail psoriasis, but also other manifestations like inflammatory bowel disease or uveitis [2]. PsA is also an immune-mediated systemic inflammatory disease associated with cardiovascular disease or events, psychological disorders and other comorbidities. As a consequence, PsA is associated with a great impact on patients’ quality of life and an increased mortality risk [3].
On the other hand, different therapeutic agents are currently available for PsA patients [4]. Therapeutic options include conventional synthetic disease-modifying antirheumatic drugs DMARDs (csDMARDs), biologic DMARDs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) including the phosphodiesterase-4 (PDE4) inhibitor apremilast, or Janus kinases (JAKs) inhibitors like tofacitinib [5]. Along with new treatments, a treat-to-target (T2T) strategy has been recommended and implemented for PsA [6]. Although there are still several major areas of ongoing unmet needs in the care of patients with PsA, an improvement in clinical outcomes has been recognised in recent years suggesting that new treatments and strategies are effective [7, 8].
In this context, randomised controlled trials (RCTs) have been central to demonstrate the efficacy of these drugs in PsA. However, most of them have analysed outcomes in the short or medium term, using mainly placebo as comparator. Direct comparative research of different drugs, important for clinical practice, is rather scarce in PsA. Besides, considering the strict inclusion criteria of RCTs or extrapolations from RA studies, study populations might not be representative of ‘real-life’ patients and, thus, results lack generalisability [9]. This issue is of key relevance in PsA taking into account the clinical heterogeneity of the disease and that comorbidities (a frequent exclusion criteria) are often present. Similarly, RCTs design and primary end-points are mainly focused on arthritis, being PsA a multifaceted domain disease.
On the other hand, consensus documents and clinical guidelines aim to analyse the best evidence to provide some guidance in treatment decision-making, even in situations where evidence is insufficient or even absent [4, 10,11,12,13]. They are usually focused on the most relevant patient’s profiles. However, in daily practice physicians have to treat patients with particular clinical situations that are not specifically covered in these documents.
Considering all of the exposed above, and to complement and try to approach some gaps in national and international guidelines, we set the following objectives (1) to define particular clinical situations, (2) to collect and analyse the best available evidence by conducting a systematic literature review (SLR), and (3) to generate practical recommendations to guide physicians in the management of multidomain complex PsA patients.
Methods
The nominal group and Delphi techniques were used. The document was generated via distribution of tasks, with the help of a systematic literature review (SLR), and under the supervision of a methodologist.
First nominal meeting group
A steering group consisting of 6 experts on PsA was established. The criteria for the selection of experts were: specialised rheumatologists in PsA, clinical experience ≥ 8 years and / or ≥ 5 publications on PsA, and members of the Spanish Society of Rheumatology (SER) and related working groups on PsA.
In the first on-line nominal meeting group, the steering group established the objectives, scope, users, definitions, and document contents. Besides, particular clinical situations in PsA were also identified. With all this information, the experts defined the inclusion and exclusion criteria for a subsequent SLR.
Systematic literature review
A SLR was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, which followed the Good Clinical Practice regulations. A protocol pertaining to the review was then designed. The research question “Which is the efficacy and safety of csDMARDs, bDMARDs and tsDMARDs in particular clinical situations in PsA” was translated to a PICO question. Studies were identified using sensitive search strategies in the main medical databases. For this purpose, an expert librarian checked the search strategies (supplementary data). Disease- and treatment-related terms were used as search keywords, which employed a controlled vocabulary, specific MeSH headings, and additional keywords. The following bibliographic databases were screened: Medline (up to March 2020), Embase (up to March 2020), and Cochrane Library (up to March 2020). Retrieved references were managed in Endnote X5 (Thomson Reuters). The abstracts of the 2018 and 2019 annual scientific meetings of the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) were also examined, along with national and international consensus documents and guidelines [4, 10,11,12,13].
Studies retrieved using the search strategies were included if they met the following pre-established criteria. Patients had to be diagnosed with PsA, aged 18 or older, with particular clinical situations, and treated with bDMARDs like tumour necrosis factor (TNF) inhibitors, abatacept, interleukin (IL)-17 inhibitors, IL-12/23 inhibitors, and ts/DMARDs like JAKs inhibitor tofacitinib, and the PDE4 inhibitor apremilast. There was no restriction regarding the type of drug, dose route of administration, concomitant use of other drugs, or treatment duration. Different outcomes were considered, such as pain, radiographic progression or quality of life. Only SLRs and RCTs in English or Spanish were included.
The screening of studies, data collection, and analysis were performed independently by two reviewers (EL and TO). In the case of discrepancy between reviewers, a consensus was reached by including a third reviewer (LC). To grade their quality, we used the Jadad score [14] for RCTs. Evidence tables were then produced, and meta-analysis was only planned if enough homogeneity (clinical and I2 ≤ 20%) among the included studies was observed.
Second nominal meeting group
The steering group participated in a second on-line meeting group. Prior to the meeting, the results of the SLR were distributed. The experts, following the results of the SLR but also based on their experience, formulated recommendations for the management of particular clinical situations in PsA.
Delphi
Recommendations were subsequently submitted to on-line Delphi voting (up to 3 rounds). Delphi was extended to a group of 65 rheumatologists (including the steering group). The participants voted each recommendation on a scale from 1 to 10 (1 = totally disagree, to 10 = totally agree). Agreement was defined if at least 70% of participants voted ≥ 7. Recommendations with a level of agreement (LA) inferior to 70% were analysed and, if appropriate, re-edited and voted in a second round.
Final consensus document
After the Delphi, and along with the results of the SLR, the final document was written. The experts agreed to focus on the approval dose of tofacitinib 5 mg bid. The methodologist assisted in assigning each recommendation, a level of evidence (LE), and grade of recommendation (GR), according to the Center for Evidence-Based Medicine of Oxford [15]. The document circulated among the experts for final assessment and comments.
Results
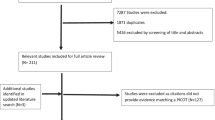
Several particular clinical situations were identified, like the presence of axial disease or dactylitis (Table 1). The SLR (designed to provide evidence for these specific patients) retrieved 181 articles of which 131 were finally included (Fig. 1). Most of them were articles of good quality. Based on their results and the experts experience and opinion, a total of 16 recommendations to guide treatment decisions in particular clinical situations. All but one reached consensus (see Table 2). The Delphi response rate was 46%.
Below, we summarise the findings of the SLR and the experts' opinion and attitude towards each particular clinical situations in PsA.
As recommended by international organisations and societies, initial treatment considerations in PsA should be based on discrete clinical manifestations, symptom severity and their impact on patients [4, 10,11,12,13]. The selection of initial treatment should also take into account comorbidities associated with PsA. And when making treatment decisions, it is important to consider that early and aggressive treatment may result in significant improvements in joint and skin symptoms, thus preventing permanent damage [16].
Articular disease (mono and oligoarthritis)
Recommendations
-
1.
Treatment and therapeutic strategy in PsA must be holistic, taking into account clinical findings and their impact on the patient's daily life (LE:5 / GR:D / LA:96%).
-
2.
In patients with monoarthritis, it is considered appropriate to start treatment with csDMARDs but also, prior to the start of csDMARDs, to consider intra-articular glucocorticoids injections or systemic short-term low-dose glucocorticoids (LE:4 / GR:C / LA:70%).
-
3.
It is recommended to consider a similar approach for oligoarthritis and polyarthritis (LE: 5 / GR:D /LA: 85%).
Evidence
The SLR found that csDMARDs are effective in PsA patients with peripheral arthritis, especially methotrexate (MTX) [17,18,19,20,21], but also other csDMARDs [22,23,24,25,26,27,28,29]. Although ciclosporin A has demonstrated efficacy [18, 30,31,32], it is usually not recommended probably due to safety issues and monitoring. bDMARDs including TNF inhibitors [30, 33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61], IL-17 inhibitors [62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] and IL-12/23 inhibitors [78,79,80,81,82] have also depicted long-term efficacy. Similarly, tsDMARDs like tofacitinib [83, 84] or apremilast [85,86,87,88,89,90] have also demonstrated efficacy.
However, specific data on patients with monoarthritis or oligoarthritis in PsA are scarce [90, 91].
Expert´s comments and contributions
The experts agreed on a holistic approach in PsA. The impact of the disease on patients’ life should always be considered. Therefore, when making treatment decisions in PsA patients with articular manifestations, apart from assessing the pattern of joint involvement, type, number and severity of affected joint/s or the presence of other manifestations, the clinical impact on patient’s daily life should be evaluated, and patients treated accordingly. As an example, in clinical practice, we might face patients with knee monoarthritis presenting more limitations than others with hand oligoarthritis. The same way, more aggressive strategies might be considered in younger patients, or treatment changes according to patient’s comorbidities.
Monoarthritis is a common manifestation in early stages of the disease and there might be patients with no other relevant manifestations. In that case, the experts equally contemplate two scenarios: cDMARDs could be started, but either intra-articular glucocorticoids injections or short-term systemic low-dose glucocorticoids prior to the start of csDMARDs could be considered [92,93,94].
Oligoarthritis is also frequent in PsA. In these patients, intra-articular / systemic glucocorticoids might not be enough to control the disease or generate adverse events. Therefore, according to the experts, oligoarthritis should be considered as a polyarthritis.
Axial disease
Recommendations
-
4.
It is recommended to select treatment according to predominant domain involvement (axial, peripheral or other) and its impact on patient (LE:5 / GR:D /LA: 96%).
-
5.
In general, in patients with axial disease, it is recommended to be cautious with the use of NSAIDs (age of patient, presence of comorbidity, etc.) (LE:1b / GR:A /LA: 92%).
-
6.
In patients with axial disease, oral or intramuscular short-term low-dose glucocorticoids (4–12 weeks) could be considered for axial symptoms (LE:4 / GR:C /LA: 23%).
Evidence
In the studies selected for the SLR, the rate of PsA patients with axial disease was generally low (< 15%) [95]. Specific sub-analyses of axial manifestations were often lacking, although some positive evidence for tofacitinib [96] or ustekinumab [97] is available. Data from observational studies are also scarce [98]. Recently, a RCT, specifically designed to assess the efficacy and safety of biological treatment for the management of axial manifestations in patients with PsA, demonstrated that secukinumab 300 and 150 mg provided significant improvement in signs and symptoms of axial disease compared with placebo [99].
On the other hand, it has been published that axial disease in PsA patients seems to be different demographically, genetically, clinically and radiographically when compared with ankylosing spondylitis (AS) with or without psoriasis [100]. Axial PsA was associated with worse peripheral arthritis and less back pain [100]. However, more research is needed to confirm these findings.
Finally, it has also been suggested that axial inflammation in patients with PsA might respond better to corticosteroids than AS patients [101]. These are preliminary data that require further studies as well.
Expert´s comments and contributions
In daily practice, PsA can present with predominant axial disease but similarly with both, axial and peripheral manifestations. Furthermore, other manifestations such as enthesitis are quite common. That is why the experts encourage rheumatologists to properly evaluate the burden and impact of each manifestation on patient’s daily life to select the most appropriate treatment. But in general, in their experience, even in patients with both axial and peripheral disease, peripheral manifestations usually generate a greater impact or disability on PsA patients.
Experts also remark that sociodemographic and clinical characteristics of PsA patients are different than AS patients. Therefore, when considering non-steroidal anti-inflammatory drugs (NSAIDs) for axial symptoms in PsA, other factors like patient’s age or the presence of comorbidities should be considered [102]. If used, the experts also prefer short courses.
As exposed, we formulated a recommendation for the evaluation of short-term low-dose glucocorticoids for axial symptoms. However, it did not reach consensus probably due to insufficient evidence and the availability of alternative effective therapies for this domain.
Enthesitis
Recommendations
-
7.
In patients with enthesitis, a careful clinical history and physical examination should be performed to rule out other non-inflammatory conditions (plantar fasciitis, trochanteric bursitis, etc.), wide-spread pain syndromes or central sensitisation, especially in patients with multiple painful entheses (LE:2a / GR:B /DA: 88%).
-
8.
In patients with enthesitis refractory to NSAIDs and/or local glucocorticoid injections, bDMARDs (except for abatacept), tofacitinib and apremilast are valid options in addition to reassess underlying inflammation with the use of imaging techniques such as ultrasound or MRI (LE:1b / DR:B /DA:92%).
Evidence
SRL reported a great variability in the enthesis evaluation of RCTs (physical examination, referred by the patient, imaging techniques, use of enthesitis indexes or counts, etc.).
Other SLRs and meta-analyses have depicted the efficacy of bDMARDs and tsDMARDs in PsA patients with enthesitis [5, 103, 104]. In in a recent meta-analysis, the estimated relative risks (RR) of enthesitis resolution in comparison to placebo across therapies were: RR = 1.99 (95% CI 1.36–2.90) for TNF inhibitors, RR = 2.31 (95% CI 1.60–3.34) for IL-17 inhibitors, RR = 1.41 (95% CI 1.02–1.95) for IL-12/23 inhibitors and RR = 0.85 (CI 0.74–0.99) for abatacept [103]. In a pooled analysis of tofacitinib, a higher rate of tofacitinib -treated patients achieved enthesitis resolution (Leed Enthesitis Index, LEI = 0) at month 3 compared with placebo. Further improvements in all enthesitis end-points were documented at month 6 [96]. Improvements have been reported with apremilast though resolution rates are modest [86, 87].
Expert´s comments and contributions
As in other sections, patient’s characteristics and impact of the disease should be a driver in the treatment decision-making.
When evaluating enthesitis, it is important to bear in mind the “enthesis organ”. It comprises the insertion of tendon and ligament to bone, but also adjacent tendon, bone fibrocartilage, fat pad, bursa, and synovium [105]. Therefore, clinical symptoms might be referred to any components of this complex and mechanical conditions, like fasciitis or bursitis, should be ruled out. Similarly, wide-spread pain syndromes or central sensitisation, especially in cases of multiple painful entheses [106], should be investigated. Finally, and based on available evidence, any bDMARD, tofacitinib or apremilast is a therapy to be considered in patients with enthesitis refractory to NSAIDs and/or local glucocorticoid injections.
Dactylitis
Recommendations
-
9.
In patients with polyarthritis, concomitant dactylitis should be treated like polyarthritis (LE:5 / GR:D / LA: 92%).
-
10.
In patients with dactylitis as predominant manifestation, before the start of csDMARDs or a treatment change, a more conservative treatment (1 or 2 local glucocorticoids injections) might be considered depending on the number of dactylitis and their impact on patient (LE:3a / GR:C / LA: 88%).
Evidence
Regarding the csDMARDs, in our SLR, MTX at 12 weeks achieved complete resolution of dactylitis in 62.7% of patients [17]. In the case of bDMARDs, TNF inhibitors have shown to be significantly superior to placebo [103], but combined therapy with csDMARD was not statistically superior to TNF inhibitor monotherapy [44, 50]. Abatacept has depicted non-statistically but numerically more rate of complete resolution of dactylitis than placebo at 24 weeks (44.3 vs 34.0%) [60]. IL-17 inhibitors secukinumab and ixekizumab (IXE) achieved significant better results than placebo in several RCTs [66, 69, 71, 77]. However, in a recent RCT (SPIRIT H2H), there were no statistically significant differences between IXE and ADA at 24 weeks in the number of patients with complete resolution of dactylitis [72]. IL-12/23 inhibitor ustekinumab data indicate a significant decrease in the number of patients with dactylitis when compared to placebo [81, 82]. In pooled analysis of 2 RCT, the proportion of patients who achieved dactylitis resolution was statistically greater for tofacitinib vs. placebo at month 3 [96]. Apremilast has also demonstrated superiority when compared to placebo [87, 89]. Nash et al. in a pooled analysis showed a significant greater change in Dactylitis Severity Score (4.6 with tofacitinib 5 mg; and 5.8 with 10 mg versus 2.5 with placebo), and percent of patients achieving dactylitis resolution (43.3% with 5 mg and 55.2% with 10 mg versus 30.6% with placebo) at month 3. Both improvements were maintained to month 6 [96].
Expert´s comments and contributions
For the experts, it is important to emphasise that dactylitis is associated with radiographic changes in PsA [107] and, therefore, must be considered as a poor prognosis factor [4]. The presence of this manifestation should be seriously considered in the treatment decision-making.
Nonetheless, in patients with dactylitis as the predominant manifestation and low number of affected fingers and impact, 1 or 2 local glucocorticoids injections might be considered before csDMARDs [108].
Skin and nail disease
-
11.
In patients with PsA and significant skin involvement, if a bDMARDs is considered, IL-17 or IL-12/23 inhibitors may be preferred (LE:1b /GR:B /DA: 88%).
Skin involvement is one of the most evaluated domains in PsA RCTs. Most of them have used Psoriasis Area and Severity Index (PASI) 75 response, but PASI 100 response or total skin clearance is increasingly being assessed [50, 72, 76]. csDMARDs (mainly MTX), b (except for abatacept) and ts/DMARDs have shown superiority compared with placebo [5]. On the other hand, nail involvement was not frequently assessed among included patients, but when evaluated the Nail Psoriasis Severity Index (NAPSI) is the preferred score. The efficacy of b (except for abatacept) and ts/DMARDs has also been reported [5, 109].
Expert´s comments and contributions
Bearing in mind the results of SLRs and meta-analyses in moderate–severe psoriasis [110], the experts consider that in patients with PsA and significant skin involvement, IL-17 or IL-12/23 inhibitors may be preferred. In these cases, treatment decision-making should be shared with patient and dermatologist.
Non-musculoskeletal manifestations and comorbidities
Recommendations
-
12.
Comorbidities should be systematically evaluated and managed in all patients with PsA (LE:5 / DR:D / DA: 92%).
Evidence
It is mandatory to point out that many RCTs exclude patients with relevant comorbidities, limiting the evidence coming from these types of articles. This is a key point since frequent comorbidities in PsA patients may influence the rheumatologist’s selection of therapy [111].
Obesity is one of the most prevalent comorbid conditions. Different studies have observed that TNF inhibitors response is inferior in obese patients. So far, this finding has not been reported with other therapies in PsA [82, 112,113,114].
Regarding cardiovascular disease, evidence is still limited, but at least csDMARDs (especially MTX) and TNF inhibitors appear to be beneficial [109, 115, 116].
Smoking is another relevant risk habit/behaviour. The DANBIO registry showed that in PsA, smokers had a poorer response to TNF inhibitors compared to non-smokers. This was most pronounced in patients treated with infliximab or etanercept [117].
On the other hand, short-term clinical trials reported a somewhat elevated risk of depression in apremilast users, resulting in a label warning. However, long-term data and real-world evidence suggest that users of apremilast had similar rates of depression compared with users of other systemic non-corticosteroid PsA drugs [118, 119]. Similar considerations can be made for suicide.
Recently, a SLR and meta-analysis have concluded that bDMARDs and apremilast had a small effect on fatigue at 24 weeks in PsA RCTs and a higher effect on pain [120]. The OPAL Beyond and OPAL Broaden RCTs also described tofacitinib efficacy in PsA patients with fatigue [83, 121].
Treatment choices for patients with concurrent PsA and inflammatory bowel disease (IBD) should be made carefully. Therapies used to treat IBD may overlap with medications used to treat PsA. Common medications for IBD that have showed efficacy are MTX, TNF inhibitors, ustekinumab and specifically for ulcerative colitis, tofacitinib [122, 123].
Finally, for patients with PsA and uveitis, csDMARDs or TNF inhibitors (except etanercept) might be preferred [124, 125].
Expert´s comments and contributions
Comorbidities in PsA are extremely relevant as clinical evaluation, treatment response and adherence to treatment could be influenced by them. Thus, comorbidities should always be evaluated.
Early PsA
Recommendations
-
13.
In patients with PsA, an early and targeted treatment strategy like TICOPA is recommended (LE:1b / GR:B / DA: 88%).
Evidence
Few studies have specifically analysed the efficacy and safety of the selected drugs in patients with early PsA [91]. Inadequate response with MTX monotherapy in this sub-group of patients has been reported [20, 126], but a RCT and open-label extension recently published observed that around half of patients with early PsA achieved remission with initial combination treatment of MTX + TNF inhibitors that was maintained with MTX monotherapy afterwards [127].
On the other hand, The TIght COntrol of inflammation in early Psoriatic Arthritis (TICOPA) study was the first strategy trial in PsA to demonstrate that a treat-to-target strategy in early disease improves clinical outcomes over a 48-week period [128, 129]. In the TICOPA study, almost 40% of patients in the tight control, treat-to-target arm, were in minimal disease activity at 48 weeks, compared with 25% in the standard of care arm. Similar experiences have been reported in an open-label study [130].
Expert´s comments and contributions
As exposed, early diagnosis and treatment of PsA is associated with better outcomes. Thus, it is highly recommended to follow TICOPA recommendations and start treatment early in the course of the disease [129].
Erosive PsA
Recommendations
-
14.
In erosive PsA, early and tight treatment and monitoring is recommended (LE:1b / GR:B / DA: 96%).
Evidence
All therapies included in the SLR have shown efficacy in radiographic progression except for abatacept, and there are no data yet for apremilast or tofacitinib versus placebo [60, 131].
Expert´s comments and contributions
PsA is associated with structural damage as a result of bone and cartilage destruction. Risk factors for radiographic progression have been identified such as elevated CRP, number of tender and swollen joints, longer disease duration, and a high current damage index [132]. Thus, the experts consider that erosions presence is a poor prognostic factor in PsA and should prompt rheumatologist to an early and tight treatment and monitoring.
Mono- vs combined therapy
Recommendations
-
15.
In patients with PsA, the use of bDMARDs, or apremilast in monotherapy or in combination with csDMARDs, or tofacitinib in combination with csDMARDs, should be individualised (LE:5 / GR:D / LA: 100%).
Evidence
Robust evidence regarding the efficacy of combined therapy compared with monotherapy in PsA is lacking. It has been depicted that MTX increases bDMARDs survival and provides a slight reduction of immunogenicity [74, 133,134,135].
Expert´s comments and contributions
The expert’s perception is that combination therapy does not provide a robust difference in efficacy in relation to monotherapy. Although commonly no significant statistical differences can be detected when disease outcomes are analysed, a tendency to favour combination therapy is observed. However, the combination might also increase the risk of adverse events. Thus, every case should be evaluated individually.
Risk management
Recommendations
-
16.
Risk management recommendations for bDMARDs, tofacitinib and apremilast from regulatory agencies and scientific societies should be followed (L:1b / GR:B / LA:100%).
Evidence
Tofacitinib should be used with caution in patients with known risk factors for venous thromboembolism. Patients should also be reassessed periodically during treatment with tofacitinib to assess changes in the risk of venous thromboembolism [136].
Along with glucocorticoids, cs/b/tsDMARD therapy is associated with an increased risk of infections [137], which is one of the most frequently reported adverse events. More specifically, herpes zoster infections have been associated with the use of systemic glucocorticoids, tofacitinib and in a lesser extent with combined therapy with TNF inhibitors + csDMARDs [5, 138, 139]. There is little information regarding other therapies. The medical board of the National Psoriasis Foundation has recently recommended the recombinant zoster vaccine for all psoriasis and PsA patients > 50 years old and patients < 50 years old on tofacitinib, systemic glucocorticoids, or combination systemic treatment. Vaccination of patients < 50 years old on other systemic therapies may be considered on a case-by-case basis [138]. Similarly, data from IL-17 inhibitors (secukinumab and IXE) RCTs and observational studies suggest and increase risk of (mild) localised candidiasis [66, 69, 70, 72, 73].
On the other hand, an increased risk of injection site reactions (mostly mild) and cases of IBD have been described with IL-17 inhibitors (secukinumab and IXE) [72, 73, 140, 141].
Lastly, different registries like PSOLAR, ARTIS or DANBIO have provided evidence that neither csDMARDs nor TNF inhibitors or ustekinumab increase the overall malignancy risk (excluding non-melanoma skin cancer) [142]. Data from IL-17 inhibitors (pooled analysis) [141], tofacitinib and apremilast are similar so far [143].
Expert´s comments and contributions
All csDMARDs, bDMARDs, tofacitinib and apremilast present adverse events, some are common across therapies, and others might be class-related adverse events. Rheumatologists should monitor and treat all of them accordingly.
Research agenda
The project also identified other issues and gaps that might be relevant for the decision-making, including pharmacogenetics data, biomarkers, basal IL levels, patient’s preferences or treatment stratification. Further research is needed to analyse their role in PsA and, therefore, in the treatment decision-making.
Discussion
This project has generated a series of recommendations to treat PsA patients focused of non-resolved definitions of particular clinical phenotypes and that remains an uncertainty source and debate in the rheumatology community. These recommendations are based on the best evidence currently available, as well as the experience of an expert team and the subsequent evaluation of a broad group of rheumatologists with experience in the management of PsA.
Despite current therapeutic armamentarium and T2T strategy for PsA patients, there is still a great variability in the clinical practice regarding to the management of patients with PsA [144]. As a consequence, different national and international societies have published recommendations for the management of these patients in recent years [4, 10,11,12,13]. However, most of these recommendations are focused on a limited definition of the target populations and might not cover all clinical scenarios for which clinicians still seek guidance. Bearing in mind those limitations, we generated specific and practical recommendations to complete those formulated in previous documents [4, 10,11,12,13].
One of our main conclusions is that given the complexity and heterogeneity of multidomain PsA disease, we should carefully assess the impact of individual manifestations on patients (disability, quality of life, etc.). Along with objective data and prognostic profile, this viewpoint might help prioritise treatment selection and strategy in a shared decision-making.
We have discussed about patients with monoarthritis who might benefit from intra-articular glucocorticoids injections or short-term systemic low-dose glucocorticoids [92,93,94]. A rapid relief may be relevant especially considering the association of monoarthritis with disability and sick leave in PsA [91]. The same principle can be applied to enthesitis or dactilytis. Besides that, the experts would like to highlight that in daily practice it is vital to manage wide-spread pain syndromes or central sensitisation, especially in patients with multiple painful entheses as these syndromes are quite frequent in PsA [106] and might lead to false diagnosis of therapeutic failure or even overtreatment. In line with current evidence (basic science and clinical data), in PsA patients with several non-musculoskeletal manifestation like uveitis, IBD or skin/nail disease, specific therapies might be preferred [110, 122,123,124,125]. The presence of certain comorbidities might negatively interfere or, the opposite, might prompt physician to select specific therapies [82, 109, 111,112,113,114,115,116,117]; thus, it should be systematically evaluated and considered in the treatment selection. Anti-IL-23 drugs (guselkumab, tildrakizumab) [145] are likely to eventually gain a place as a therapeutic target, when experience is gained in different profiles and efficacy and safety are proven in real life. Finally, we also encourage physicians to follow T2T strategies in early PsA as they are providing promising results [128,129,130].
On the other hand, the main limitation of this work is the lack of published quality evidence that specifically address some of the open questions on the selected clinical phenotypes. For this reason, expert opinion is the only way to deliver recommendations that try to resolve uncertainty. In this regard, a strength of the study is the broad evaluation of the set recommendations that was extended to a significant number of rheumatologists through a Delphi process. A very high level of agreement in all but one recommendation was reached, which increases the validity of the recommendations.
In conclusion, treatment decisions are not always straightforward in PsA. We are confident that these recommendations will find their way into the clinic for a better care of the PsA patients in the real-world setting.
References
Liew JW, Ramiro S, Gensler LS (2018) Cardiovascular morbidity and mortality in ankylosing spondylitis and psoriatic arthritis. Best Pract Res Clin Rheumatol 32(3):369–389. https://doi.org/10.1016/j.berh.2019.01.002
Pittam B, Gupta S, Harrison NL, Robertson S, Hughes DM, Zhao SS (2020) Prevalence of extra-articular manifestations in psoriatic arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 59(9):2199–2206. https://doi.org/10.1093/rheumatology/keaa062
Gupta S, Syrimi Z, Hughes DM, Zhao SS (2021) Comorbidities in psoriatic arthritis: a systematic review and meta-analysis. Rheumatol Int 41(2):275–284. https://doi.org/10.1007/s00296-020-04775-2
Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, Primdahl J, McGonagle DG, Aletaha D, Balanescu A, Balint PV, Bertheussen H, Boehncke WH, Burmester GR, Canete JD, Damjanov NS, Kragstrup TW, Kvien TK, Landewe RBM, Lories RJU, Marzo-Ortega H, Poddubnyy D, Rodrigues Manica SA, Schett G, Veale DJ, Van den Bosch FE, van der Heijde D, Smolen JS (2020) EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 79(6):700–712. https://doi.org/10.1136/annrheumdis-2020-217159
Kerschbaumer A, Smolen JS, Dougados M, de Wit M, Primdahl J, McInnes I, van der Heijde D, Baraliakos X, Falzon L, Gossec L (2020) Pharmacological treatment of psoriatic arthritis: a systematic literature research for the 2019 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 79(6):778–786. https://doi.org/10.1136/annrheumdis-2020-217163
Smolen JS, Schols M, Braun J, Dougados M, FitzGerald O, Gladman DD, Kavanaugh A, Landewe R, Mease P, Sieper J, Stamm T, Wit M, Aletaha D, Baraliakos X, Betteridge N, Bosch FVD, Coates LC, Emery P, Gensler LS, Gossec L, Helliwell P, Jongkees M, Kvien TK, Inman RD, McInnes IB, Maccarone M, Machado PM, Molto A, Ogdie A, Poddubnyy D, Ritchlin C, Rudwaleit M, Tanew A, Thio B, Veale D, Vlam K, van der Heijde D (2018) Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 77(1):3–17. https://doi.org/10.1136/annrheumdis-2017-211734
Haugeberg G, Michelsen B, Tengesdal S, Hansen IJW, Diamantopoulos A, Kavanaugh A (2018) Ten years of follow-up data in psoriatic arthritis: results based on standardized monitoring of patients in an ordinary outpatient clinic in southern Norway. Arthritis Res Ther 20(1):160. https://doi.org/10.1186/s13075-018-1659-z
Colaco K, Widdifield J, Luo J, Rosen CF, Alhusayen R, Paterson JM, Campbell W, Tu K, Bernatsky S, Gladman DD, Eder L (2020) Trends in mortality and cause-specific mortality among patients with psoriasis and psoriatic arthritis in Ontario, Canada. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.10.031
Vashisht P, Sayles H, Cannella AC, Mikuls TR, Michaud K (2016) Generalizability of patients with rheumatoid arthritis in biologic agent clinical trials. Arthritis Care Res (Hoboken) 68(10):1478–1488. https://doi.org/10.1002/acr.22860
Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, Bautista-Molano W, Boehncke WH, Campbell W, Cauli A, Espinoza LR, FitzGerald O, Gladman DD, Gottlieb A, Helliwell PS, Husni ME, Love TJ, Lubrano E, McHugh N, Nash P, Ogdie A, Orbai AM, Parkinson A, O’Sullivan D, Rosen CF, Schwartzman S, Siegel EL, Toloza S, Tuong W, Ritchlin CT (2016) Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 68(5):1060–1071. https://doi.org/10.1002/art.39573
Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, Dubreuil M, Dunham J, Husni ME, Kenny S, Kwan-Morley J, Lin J, Marchetta P, Mease PJ, Merola JF, Miner J, Ritchlin CT, Siaton B, Smith BJ, Van Voorhees AS, Jonsson AH, Shah AA, Sullivan N, Turgunbaev M, Coates LC, Gottlieb A, Magrey M, Nowell WB, Orbai AM, Reddy SM, Scher JU, Siegel E, Siegel M, Walsh JA, Turner AS, Reston J (2019) Special article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 71(1):5–32. https://doi.org/10.1002/art.40726
Torre Alonso JC, Del Campo D, Fontecha P, Almodovar R, Canete JD, Montilla Morales C, Moreno M, Plasencia-Rodriguez C, Ramirez Garcia J, Queiro R (2018) Recommendations of the Spanish Society of Rheumatology on treatment and use of systemic biological and non-biological therapies in psoriatic arthritis. Reumatol Clin 14(5):254–268. https://doi.org/10.1016/j.reuma.2017.08.007
Ritchlin CT, Kavanaugh A, Gladman DD, Mease PJ, Helliwell P, Boehncke WH, de Vlam K, Fiorentino D, Fitzgerald O, Gottlieb AB, McHugh NJ, Nash P, Qureshi AA, Soriano ER, Taylor WJ, Group for R, Assessment of P, Psoriatic A (2009) Treatment recommendations for psoriatic arthritis. Ann Rheum Dis 68(9):1387–1394. https://doi.org/10.1136/ard.2008.094946
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
(CEBM) OUCfE-BM (2011) CEBM Levels of Evidence 2011. https://www.cebm.net/. Accessed 29 Sept 2020
Merola JF, Lockshin B, Mody EA (2017) Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum 47(1):29–37. https://doi.org/10.1016/j.semarthrit.2017.02.001
Coates LC, Helliwell PS (2016) Methotrexate efficacy in the tight control in psoriatic arthritis study. J Rheumatol 43(2):356–361. https://doi.org/10.3899/jrheum.150614
Fraser AD, van Kuijk AW, Westhovens R, Karim Z, Wakefield R, Gerards AH, Landewé R, Steinfeld SD, Emery P, Dijkmans BA et al (2005) A randomised, double blind, placebo controlled, multicentre trial of combination therapy with methotrexate plus ciclosporin in patients with active psoriatic arthritis. Ann Rheum Dis 64(6):859–864. https://doi.org/10.1136/ard.2004.024463
Kingsley GH, Kowalczyk A, Taylor H, Ibrahim F, Packham JC, McHugh NJ, Mulherin DM, Kitas GD, Chakravarty K, Tom BD et al (2012) A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford) 51(8):1368–1377. https://doi.org/10.1093/rheumatology/kes001
Scarpa R, Peluso R, Atteno M, Manguso F, Spanò A, Iervolino S, Di Minno MN, Costa L, Del Puente A (2008) The effectiveness of a traditional therapeutical approach in early psoriatic arthritis: results of a pilot randomised 6-month trial with methotrexate. Clin Rheumatol 27(7):823–826. https://doi.org/10.1007/s10067-007-0787-7
Willkens RF, Williams HJ, Ward JR, Egger MJ, Reading JC, Clements PJ, Cathcart ES, Samuelson CO Jr, Solsky MA, Kaplan SB (1984) Randomized, double-blind, placebo controlled trial of low-dose pulse methotrexate in psoriatic arthritis. Arthritis Rheum 27(4):376–381. https://doi.org/10.1002/art.1780270403
Clegg DO, Reda DJ, Mejias E, Cannon GW, Weisman MH, Taylor T, Budiman-Mak E, Blackburn WD, Vasey FB, Mahowald ML et al (1996) Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A Department of Veterans Affairs Cooperative Study. Arthritis Rheum 39(12):2013–2020. https://doi.org/10.1002/art.1780391210
Combe B, Goupille P, Kuntz JL, Tebib J, Lioté F, Bregeon C (1996) Sulphasalazine in psoriatic arthritis: a randomized, multicentre, placebo-controlled study. Br J Rheumatol 35(7):664–668. https://doi.org/10.1093/rheumatology/35.7.664
Dougados M, vam der Linden S, Leirisalo-Repo M, Huitfeldt B, Juhlin R, Veys E, Zeidler H, Kvien TK, Olivieri I, Dijkmans B (1995) Sulfasalazine in the treatment of spondylarthropathy. A randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheum 38(5):618–627. https://doi.org/10.1002/art.1780380507
Fraser SM, Hopkins R, Hunter JA, Neumann V, Capell HA, Bird HA (1993) Sulphasalazine in the management of psoriatic arthritis. Br J Rheumatol 32(10):923–925. https://doi.org/10.1093/rheumatology/32.10.923
Kaltwasser JP, Nash P, Gladman D, Rosen CF, Behrens F, Jones P, Wollenhaupt J, Falk FG, Mease P (2004) Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum 50(6):1939–1950. https://doi.org/10.1002/art.20253
Nash P, Thaçi D, Behrens F, Falk F, Kaltwasser JP (2006) Leflunomide improves psoriasis in patients with psoriatic arthritis: an in-depth analysis of data from the TOPAS study. Dermatology (Basel, switzerland) 212(3):238–249. https://doi.org/10.1159/000091251
Brückle W, Dexel T, Grasedyck K, Schattenkirchner M (1994) Treatment of psoriatic arthritis with auranofin and gold sodium thiomalate. Clin Rheumatol 13(2):209–216. https://doi.org/10.1007/bf02249014
Palit J, Hill J, Capell HA, Carey J, Daunt SO, Cawley MI, Bird HA, Nuki G (1990) A multicentre double-blind comparison of auranofin, intramuscular gold thiomalate and placebo in patients with psoriatic arthritis. Br J Rheumatol 29(4):280–283. https://doi.org/10.1093/rheumatology/29.4.280
Atzeni F, Boccassini L, Antivalle M, Salaffi F, Sarzi-Puttini P (2011) Etanercept plus ciclosporin versus etanercept plus methotrexate for maintaining clinical control over psoriatic arthritis: a randomised pilot study. Ann Rheum Dis 70(4):712–714. https://doi.org/10.1136/ard.2010.130864
Spadaro A, Riccieri V, Sili-Scavalli A, Sensi F, Taccari E, Zoppini A (1995) Comparison of cyclosporin A and methotrexate in the treatment of psoriatic arthritis: a one-year prospective study. Clin Exp Rheumatol 13(5):589–593
Spadaro A, Taccari E, Sensi F, Riccieri V, Sili Scavalli A, Zoppini A (1998) Soluble interleukin-2 receptor and interleukin-6 levels: evaluation during cyclosporin A and methotrexate treatment in psoriatic arthritis. Clin Rheumatol 17(1):83–85. https://doi.org/10.1007/bf01450970
Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, Zhou B, Dooley LT, Kavanaugh A, Investigators IT (2005) Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 64(8):1150–1157. https://doi.org/10.1136/ard.2004.032268
Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, Furst DE, Molitor J, Keystone E, Gladman D, Manger B, Wassenberg S, Weier R, Wallace DJ, Weisman MH, Kalden JR, Smolen J (2005) Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 52(4):1227–1236. https://doi.org/10.1002/art.20967
Antoni CE, Kavanaugh A, van der Heijde D, Beutler A, Keenan G, Zhou B, Kirkham B, Tutuncu Z, Burmester GR, Schneider U et al (2008) Two-year efficacy and safety of infliximab treatment in patients with active psoriatic arthritis: findings of the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT). J Rheumatol 35(5):869–876
Baranauskaite A, Raffayová H, Kungurov NV, Kubanova A, Venalis A, Helmle L, Srinivasan S, Nasonov E, Vastesaeger N (2012) Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND study. Ann Rheum Dis 71(4):541–548. https://doi.org/10.1136/ard.2011.152223
Jørgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, Lundin KEA, Mørk C, Jahnsen J, Kvien TK (2017) Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet (London, England) 389(10086):2304–2316. https://doi.org/10.1016/S0140-6736(17)30068-5
Kavanaugh A, Krueger GG, Beutler A, Guzzo C, Zhou B, Dooley LT, Mease PJ, Gladman DD, de Vlam K, Geusens PP et al (2007) Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis 66(4):498–505. https://doi.org/10.1136/ard.2006.058339
Genovese MC, Mease PJ, Thomson GT, Kivitz AJ, Perdok RJ, Weinberg MA, Medich J, Sasso EH (2007) Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J Rheumatol 34(5):1040–1050
Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, Sharp JT, Ory PA, Perdok RJ, Weinberg MA (2005) Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 52(10):3279–3289. https://doi.org/10.1002/art.21306
Mease PJ, Heckaman M, Kary S, Kupper H (2013) Application and modifications of minimal disease activity measures for patients with psoriatic arthritis treated with adalimumab: subanalyses of ADEPT. J Rheumatol 40(5):647–652. https://doi.org/10.3899/jrheum.120970
Gniadecki R, Robertson D, Molta CT, Freundlich B, Pedersen R, Li W, Boggs R, Zbrozek AS (2012) Self-reported health outcomes in patients with psoriasis and psoriatic arthritis randomized to two etanercept regimens. J Eur Acad Dermatol Venereol 26(11):1436–1443. https://doi.org/10.1111/j.1468-3083.2011.04308.x
Kirkham B, de Vlam K, Li W, Boggs R, Mallbris L, Nab HW, Tarallo M (2015) Early treatment of psoriatic arthritis is associated with improved patient-reported outcomes: findings from the etanercept PRESTA trial. Clin Exp Rheumatol 33(1):11–19
Mease PJ, Gladman DD, Collier DH, Ritchlin CT, Helliwell PS, Liu L, Kricorian G, Chung JB (2019) Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol (Hoboken, NJ) 71(7):1112–1124. https://doi.org/10.1002/art.40851
Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ (2000) Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet (London, England) 356(9227):385–390. https://doi.org/10.1016/S0140-6736(00)02530-7
Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, Salonen D, Rubenstein J, Sharp JT, Dunn M et al (2006) Continued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanercept. J Rheumatol 33(4):712–721
Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, Salonen D, Rubenstein J, Sharp JT, Tsuji W (2004) Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 50(7):2264–2272. https://doi.org/10.1002/art.20335
Sterry W, Ortonne JP, Kirkham B, Brocq O, Robertson D, Pedersen RD, Estojak J, Molta CT, Freundlich B (2010) Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ (Clinical Res Ed) 340:c147. https://doi.org/10.1136/bmj.c147
Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, Woltering F, Stach C, Hoepken B, Arledge T et al (2014) Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 73(1):48–55. https://doi.org/10.1136/annrheumdis-2013-203696
Walsh JA, Gottlieb AB, Hoepken B, Nurminen T, Mease PJ (2018) Efficacy of certolizumab pegol with and without concomitant use of disease-modifying anti-rheumatic drugs over 4 years in psoriatic arthritis patients: results from the RAPID-PsA randomized controlled trial. Clin Rheumatol 37(12):3285–3296. https://doi.org/10.1007/s10067-018-4227-7
Carron P, Varkas G, Cypers H, Van Praet L, Elewaut D, Van den Bosch F (2017) Anti-TNF-induced remission in very early peripheral spondyloarthritis: the CRESPA study. Ann Rheum Dis 76(8):1389–1395. https://doi.org/10.1136/annrheumdis-2016-210775
Kavanaugh A, Husni ME, Harrison DD, Kim L, Lo KH, Leu JH, Hsia EC (2017) Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results Through Week Twenty-Four of the GO-VIBRANT Study. Arthritis Rheumatol (Hoboken, NJ) 69(11):2151–2161. https://doi.org/10.1002/art.40226
Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, Papp K, Zrubek J, Mudivarthy S, Mack M et al (2009) Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 60(4):976–986. https://doi.org/10.1002/art.24403
Kavanaugh A, McInnes IB, Krueger GG, Gladman D, Beutler A, Gathany T, Mack M, Tandon N, Han C, Mease P (2013) Patient-reported outcomes and the association with clinical response in patients with active psoriatic arthritis treated with golimumab: findings through 2 years of a phase III, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Care Res 65(10):1666–1673. https://doi.org/10.1002/acr.22044
Kavanaugh A, McInnes IB, Mease P, Krueger GG, Gladman D, van der Heijde D, Zhou Y, Lu J, Leu JH, Goldstein N et al (2014) Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann Rheum Dis 73(9):1689–1694. https://doi.org/10.1136/annrheumdis-2013-204902
Kavanaugh A, van der Heijde D, Beutler A, Gladman D, Mease P, Krueger GG, McInnes IB, Helliwell P, Coates LC, Xu S (2016) Radiographic progression of patients with psoriatic arthritis who achieve minimal disease activity in response to golimumab therapy: results through 5 years of a randomized, placebo-controlled study. Arthritis Care Res 68(2):267–274. https://doi.org/10.1002/acr.22576
Kavanaugh A, van der Heijde D, McInnes IB, Mease P, Krueger GG, Gladman DD, Gómez-Reino J, Papp K, Baratelle A, Xu W et al (2012) Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum 64(8):2504–2517. https://doi.org/10.1002/art.34436
van Mens LJJ, de Jong HM, Fluri I, Nurmohamed MT, van de Sande MGH, Kok M, van Kuijk AWR, Baeten D (2019) Achieving remission in psoriatic arthritis by early initiation of TNF inhibition: a double-blind, randomised, placebo-controlled trial of golimumab plus methotrexate versus placebo plus methotrexate. Ann Rheum Dis 78(5):610–616. https://doi.org/10.1136/annrheumdis-2018-214746
Genovese MC, Weinblatt ME, Mease PJ, Aelion JA, Peloso PM, Chen K, Li Y, Liu J, Othman AA, Khatri A et al (2018) Dual inhibition of tumour necrosis factor and interleukin-17A with ABT-122: open-label long-term extension studies in rheumatoid arthritis or psoriatic arthritis. Rheumatology (Oxford) 57(11):1972–1981. https://doi.org/10.1093/rheumatology/key173
Mease PJ, Gottlieb AB, van der Heijde D, FitzGerald O, Johnsen A, Nys M, Banerjee S, Gladman DD (2017) Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis 76(9):1550–1558. https://doi.org/10.1136/annrheumdis-2016-210724
Strand V, Alemao E, Lehman T, Johnsen A, Banerjee S, Ahmad HA, Mease PJ (2018) Improved patient-reported outcomes in patients with psoriatic arthritis treated with abatacept: results from a phase 3 trial. Arthritis Res Ther 20(1):269. https://doi.org/10.1186/s13075-018-1769-7
Coates LC, Gladman DD, Nash P, FitzGerald O, Kavanaugh A, Kvien TK, Gossec L, Strand V, Rasouliyan L, Pricop L et al (2018) Secukinumab provides sustained PASDAS-defined remission in psoriatic arthritis and improves health-related quality of life in patients achieving remission: 2-year results from the phase III FUTURE 2 study. Arthritis Res Ther 20(1):272. https://doi.org/10.1186/s13075-018-1773-y
Coates LC, Mease PJ, Gossec L, Kirkham B, Sherif B, Gaillez C, Mpofu S, Jugl SM, Karyekar C, Gandhi KK (2018) Minimal disease activity among active psoriatic arthritis patients treated with secukinumab: 2-year results from a multicenter, randomized, double-blind, parallel-group, placebo-controlled phase III study. Arthritis Care Res 70(10):1529–1535. https://doi.org/10.1002/acr.23537
Kavanaugh A, McInnes IB, Mease PJ, Hall S, Chinoy H, Kivitz AJ, Wang Z, Mpofu S (2016) Efficacy of subcutaneous secukinumab in patients with active psoriatic arthritis stratified by prior tumor necrosis factor inhibitor use: results from the randomized placebo-controlled FUTURE 2 study. J Rheumatol 43(9):1713–1717. https://doi.org/10.3899/jrheum.160275
Kavanaugh A, Mease PJ, Reimold AM, Tahir H, Rech J, Hall S, Geusens P, Wang Z, Pricop L, Mpofu S (2017) Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res 69(3):347–355. https://doi.org/10.1002/acr.23111
McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, van der Heijde D, Landewé R, Conaghan PG, Gottlieb AB et al (2015) Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England) 386(9999):1137–1146. https://doi.org/10.1016/S0140-6736(15)61134-5
McInnes IB, Mease PJ, Ritchlin CT, Rahman P, Gottlieb AB, Kirkham B, Kajekar R, Delicha EM, Pricop L, Mpofu S (2017) Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology (Oxford) 56(11):1993–2003. https://doi.org/10.1093/rheumatology/kex301
McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, Dahmen G, Wollenhaupt J, Schulze-Koops H, Kogan J et al (2014) Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis 73(2):349–356. https://doi.org/10.1136/annrheumdis-2012-202646
Mease P, van der Heijde D, Landewé R, Mpofu S, Rahman P, Tahir H, Singhal A, Boettcher E, Navarra S, Meiser K et al (2018) Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis 77(6):890–897. https://doi.org/10.1136/annrheumdis-2017-212687
Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, Landewé R, Nash P, Pricop L, Yuan J et al (2015) Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med 373(14):1329–1339. https://doi.org/10.1056/NEJMoa1412679
Nash P, Mease PJ, McInnes IB, Rahman P, Ritchlin CT, Blanco R, Dokoupilova E, Andersson M, Kajekar R, Mpofu S et al (2018) Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther 20(1):47. https://doi.org/10.1186/s13075-018-1551-x
Mease PJ, Smolen JS, Behrens F, Nash P, Liu Leage S, Li L, Tahir H, Gooderham M, Krishnan E, Liu-Seifert H, Emery P, Pillai SG, Helliwell PS, group SHHs, (2020) A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naive patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis 79(1):123–131. https://doi.org/10.1136/annrheumdis-2019-215386
Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, Lin CY, Braun DK, Lee CH, Gladman DD (2017) Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 76(1):79–87. https://doi.org/10.1136/annrheumdis-2016-209709
Nash P, Behrens F, Orbai AM, Rathmann SS, Adams DH, Benichou O, Deodhar A (2018) Ixekizumab is efficacious when used alone or when added to conventional synthetic disease-modifying antirheumatic drugs (cDMARDs) in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor inhibitors. RMD Open. https://doi.org/10.1136/rmdopen-2018-000692
Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester GR, Adams DH, Kerr L, Lee C, Shuler CL et al (2017) Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet (london, england) 389(10086):2317–2327. https://doi.org/10.1016/S0140-6736(17)31429-0
van der Heijde D, Gladman DD, Kishimoto M, Okada M, Rathmann SS, Moriarty SR, Shuler CL, Carlier H, Benichou O, Mease PJ (2018) Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol 45(3):367–377. https://doi.org/10.3899/jrheum.170429
Genovese MC, Combe B, Kremer JM, Tsai TF, Behrens F, Adams DH, Lee C, Kerr L, Nash P (2018) Safety and efficacy of ixekizumab in patients with PsA and previous inadequate response to TNF inhibitors: week 52 results from SPIRIT-P2. Rheumatology (Oxford) 57(11):2001–2011. https://doi.org/10.1093/rheumatology/key182
Araujo EG, Englbrecht M, Hoepken S, Finzel S, Kampylafka E, Kleyer A, Bayat S, Schoenau V, Hueber A, Rech J et al (2019) Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin Arthritis Rheum 48(4):632–637. https://doi.org/10.1016/j.semarthrit.2018.05.011
Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, Fretzin S, Kunynetz R, Kavanaugh A (2009) Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet (London, England) 373(9664):633–640. https://doi.org/10.1016/S0140-6736(09)60140-9
Kavanaugh A, Puig L, Gottlieb AB, Ritchlin C, Li S, Wang Y, Mendelsohn AM, Song M, Zhu Y, Rahman P et al (2015) Maintenance of clinical efficacy and radiographic benefit through two years of ustekinumab therapy in patients with active psoriatic arthritis: results from a randomized, placebo-controlled phase III trial. Arthritis Care Res 67(12):1739–1749. https://doi.org/10.1002/acr.22645
McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM et al (2013) Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet (London, England) 382(9894):780–789. https://doi.org/10.1016/S0140-6736(13)60594-2
Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, Wang Y, Shen YK, Doyle MK, Mendelsohn AM et al (2014) Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 73(6):990–999. https://doi.org/10.1136/annrheumdis-2013-204655
Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, Kudlacz E, Wang C, Menon S, Hendrikx T et al (2017) Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 377(16):1525–1536. https://doi.org/10.1056/NEJMoa1615977
Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, Cieślak D, Graham D, Wang C, Menon S et al (2017) Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 377(16):1537–1550. https://doi.org/10.1056/NEJMoa1615975
Cutolo M, Myerson GE, Fleischmann RM, Lioté F, Díaz-González F, Van den Bosch F, Marzo-Ortega H, Feist E, Shah K, Hu C et al (2016) A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 Trial. J Rheumatol 43(9):1724–1734. https://doi.org/10.3899/jrheum.151376
Edwards CJ, Blanco FJ, Crowley J, Birbara CA, Jaworski J, Aelion J, Stevens RM, Vessey A, Zhan X, Bird P (2016) Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis 75(6):1065–1073. https://doi.org/10.1136/annrheumdis-2015-207963
Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, Hochfeld M, Teng LL, Schett G, Lespessailles E et al (2015) Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol 42(3):479–488. https://doi.org/10.3899/jrheum.140647
Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, Lespessailles E, Hall S, Hochfeld M, Hu C et al (2014) Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis 73(6):1020–1026. https://doi.org/10.1136/annrheumdis-2013-205056
Nash P, Ohson K, Walsh J, Delev N, Nguyen D, Teng L, Gómez-Reino JJ, Aelion JA (2018) Early and sustained efficacy with apremilast monotherapy in biological-naïve patients with psoriatic arthritis: a phase IIIB, randomised controlled trial (ACTIVE). Ann Rheum Dis 77(5):690–698. https://doi.org/10.1136/annrheumdis-2017-211568
Schett G, Wollenhaupt J, Papp K, Joos R, Rodrigues JF, Vessey AR, Hu C, Stevens R, de Vlam KL (2012) Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 64(10):3156–3167. https://doi.org/10.1002/art.34627
Kasiem FR, Luime JJ, Vis M, Kok MR, Wervers K, Gerards AH, Appels C, van der Graaff WL, Starmans-Kool M, Goekoop-Ruiterman Y, van Groenendael J, Korswagen LA, Veris-van Dieren JJ, Hazes J (2020) Tchetverikov I (2020) Lessons learned from clinical phenotypes in early psoriatic arthritis: the real-world Dutch south west Early Psoriatic ARthritis study. Scand J Rheumatol 10(1080/03009742):1803398
Abji F, Ye JY, Cook RJ, Oikonomopoulou K, Chandran V (2020) The association between synovial fluid serine proteinase activity and response to intra-articular corticosteroid injection in psoriatic arthritis. Clin Rheumatol 39(8):2355–2361. https://doi.org/10.1007/s10067-020-05003-9
Carubbi F, Zugaro L, Cipriani P, Conchiglia A, Gregori L, Danniballe C, Letizia Pistoia M, Liakouli V, Ruscitti P, Ciccia F, Triolo G, Masciocchi C, Giacomelli R (2016) Safety and efficacy of intra-articular anti-tumor necrosis factor α agents compared to corticosteroids in a treat-to-target strategy in patients with inflammatory arthritis and monoarthritis flare. Int J Immunopathol Pharmacol 29(2):252–266. https://doi.org/10.1177/0394632015593220
Eder L, Chandran V, Ueng J, Bhella S, Lee KA, Rahman P, Pope A, Cook RJ, Gladman DD (2010) Predictors of response to intra-articular steroid injection in psoriatic arthritis. Rheumatology (Oxford) 49(7):1367–1373. https://doi.org/10.1093/rheumatology/keq102
Ureyen SB, Ivory C, Kalyoncu U, Karsh J, Aydin SZ (2018) What does evidence-based medicine tell us about treatments for different subtypes of psoriatic arthritis? A systematic literature review on randomized controlled trials. Rheumatol Adv Practice 2(1):1–15. https://doi.org/10.1093/rap/rkx019
Nash P, Coates LC, Fleischmann R, Papp KA, Gomez-Reino JJ, Kanik KS, Wang C, Wu J, Menon S, Hendrikx T, Ports WC (2018) Efficacy of tofacitinib for the treatment of psoriatic arthritis: pooled analysis of two phase 3 studies. Rheumatol Ther 5(2):567–582. https://doi.org/10.1007/s40744-018-0131-5
Kavanaugh A, Puig L, Gottlieb AB, Ritchlin C, You Y, Li S, Song M, Randazzo B, Rahman P, McInnes IB (2016) Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis 75(11):1984–1988. https://doi.org/10.1136/annrheumdis-2015-209068
Lubrano E, Spadaro A, Marchesoni A, Olivieri I, Scarpa R, D’Angelo S, Salvarani C, Mathieu A, Cauli A, Ferrara N, Helliwell P (2011) The effectiveness of a biologic agent on axial manifestations of psoriatic arthritis. A twelve months observational study in a group of patients treated with etanercept. Clin Exp Rheumatol 29(1):80–84
Baraliakos X, Gossec L, Pournara E, Jeka S, Mera-Varela A, D’Angelo S, Schulz B, Rissler M, Nagar K, Perella C, Coates LC (2020) Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2020-218808
Feld J, Ye JY, Chandran V, Inman RD, Haroon N, Cook R, Gladman DD (2020) Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? Rheumatology (Oxford) 59(6):1340–1346. https://doi.org/10.1093/rheumatology/kez457
Haroon M, Ahmad M, Baig MN, Mason O, Rice J, FitzGerald O (2018) Inflammatory back pain in psoriatic arthritis is significantly more responsive to corticosteroids compared to back pain in ankylosing spondylitis: a prospective, open-labelled, controlled pilot study. Arthritis Res Ther 20(1):73. https://doi.org/10.1186/s13075-018-1565-4
Ash Z, Gaujoux-Viala C, Gossec L, Hensor EMA, FitzGerald O, Winthrop K, van der Heijde D, Emery P, Smolen JS, Marzo-Ortega H (2012) A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 71(3):319–326. https://doi.org/10.1136/ard.2011.150995
Simons N, Degboe Y, Barnetche T, Cantagrel A, Ruyssen-Witrand A, Constantin A (2020) Biological DMARD efficacy in psoriatic arthritis: a systematic literature review and meta-analysis on articular, enthesitis, dactylitis, skin and functional outcomes. Clin Exp Rheumatol 38(3):508–515
Gratacos Masmitja J, Gonzalez Fernandez CM, Gomez Castro S, Rebollo Laserna FJ (2020) Efficacy of tofacitinib in the treatment of psoriatic arthritis: a systematic review. Adv Ther. https://doi.org/10.1007/s12325-020-01585-7
Ritchlin CT (2014) Psoriatic enthesitis: an update from the GRAPPA 2013 Annual Meeting. J Rheumatol 41(6):1220–1223. https://doi.org/10.3899/jrheum.140174
Marchesoni A, De Marco G, Merashli M, McKenna F, Tinazzi I, Marzo-Ortega H, McGonagle DG (2018) The problem in differentiation between psoriatic-related polyenthesitis and fibromyalgia. Rheumatology (Oxford) 57(1):32–40. https://doi.org/10.1093/rheumatology/kex079
Geijer M, Lindqvist U, Husmark T, Alenius GM, Larsson PT, Teleman A, Theander E (2015) The Swedish early psoriatic arthritis registry 5-year followup: substantial radiographic progression mainly in men with high disease activity and development of dactylitis. J Rheumatol 42(11):2110–2117. https://doi.org/10.3899/jrheum.150165
Girolimetto N, Macchioni P, Citriniti G, Tinazzi I, Bascherini V, Martinis F, Marchetta A, Possemato N, Tasso M, Peluso R, Punzi L, Salvarani C, Scarpa R, McGonagle D, Costa L, Caso F (2020) Effectiveness of steroid injection for hand psoriatic dactylitis: results from a multicentre prospective observational study. Clin Rheumatol 39(11):3383–3392. https://doi.org/10.1007/s10067-020-05142-z
Armstrong AW, Brezinski EA, Follansbee MR, Armstrong EJ (2014) Effects of biologic agents and other disease-modifying antirheumatic drugs on cardiovascular outcomes in psoriasis and psoriatic arthritis: a systematic review. Curr Pharm Des 20(4):500–512. https://doi.org/10.2174/138161282004140213123505
Sbidian E, Chaimani A, Afach S, Doney L, Dressler C, Hua C, Mazaud C, Phan C, Hughes C, Riddle D, Naldi L, Garcia-Doval I, Le Cleach L (2020) Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev 1:CD011535. https://doi.org/10.1002/14651858.CD011535.pub3
Gupta S, Syrimi Z, Hughes DM, Zhao SS (2021) Comorbidities in psoriatic arthritis: a systematic review and meta-analysis. Rheumatol Int. https://doi.org/10.1007/s00296-020-04775-2
Di Minno MN, Peluso R, Iervolino S, Russolillo A, Lupoli R, Scarpa R (2014) Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor α blockers. Ann Rheum Dis 73(6):1157–1162. https://doi.org/10.1136/annrheumdis-2012-202812
Galíndez E, Carmona L (2016) Is obesity in psoriatic arthritis associated with a poorer therapeutic response and more adverse effects of treatment with an anchor drug? Reumatologia Clinica 12(6):307–312. https://doi.org/10.1016/j.reuma.2015.12.005
McInnes IB, Ferraccioli G, D’Agostino MA, Le Bars M, Banerjee S, Ahmad HA, Elbez Y, Mease PJ (2019) Body mass index and treatment response to subcutaneous abatacept in patients with psoriatic arthritis: a post hoc analysis of a phase III trial. RMD Open 5(1):e000934. https://doi.org/10.1136/rmdopen-2019-000934
Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, Gulliver W, Keeling S, Dutz J, Bessette L, Bissonnette R, Haraoui B (2015) The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 74(3):480–489. https://doi.org/10.1136/annrheumdis-2014-206624
Persson R, Hagberg KW, Qian Y, Vasilakis-Scaramozza C, Jick S (2021) The risks of major cardiac events among patients with psoriatic arthritis treated with apremilast, biologics, DMARDs or corticosteroids. Rheumatology (Oxford) 60(4):1926–1931. https://doi.org/10.1093/rheumatology/keaa683
Hojgaard P, Glintborg B, Hetland ML, Hansen TH, Lage-Hansen PR, Petersen MH, Holland-Fischer M, Nilsson C, Loft AG, Andersen BN, Adelsten T, Jensen J, Omerovic E, Christensen R, Tarp U, Ostgard R, Dreyer L (2015) Association between tobacco smoking and response to tumour necrosis factor alpha inhibitor treatment in psoriatic arthritis: results from the DANBIO registry. Ann Rheum Dis 74(12):2130–2136. https://doi.org/10.1136/annrheumdis-2014-205389
Mease PJ, Gladman DD, Gomez-Reino JJ, Hall S, Kavanaugh A, Lespessailles E, Schett G, Paris M, Delev N, Teng L, Wollenhaupt J (2020) Long-term safety and tolerability of apremilast versus placebo in psoriatic arthritis: a pooled safety analysis of three phase III, randomized, controlled trials. ACR Open Rheumatol 2(8):459–470. https://doi.org/10.1002/acr2.11156
Vasilakis-Scaramozza C, Persson R, Hagberg KW, Jick S (2020) The risk of treated anxiety and treated depression among patients with psoriasis and psoriatic arthritis treated with apremilast compared to biologics, DMARDs and corticosteroids: a cohort study in the United States MarketScan database. J Eur Acad Dermatol Venereol 34(8):1755–1763. https://doi.org/10.1111/jdv.16231
Reygaerts T, Mitrovic S, Fautrel B, Gossec L (2018) Effect of biologics on fatigue in psoriatic arthritis: a systematic literature review with meta-analysis. Joint Bone Spine 85(4):405–410. https://doi.org/10.1016/j.jbspin.2018.01.011
Strand V, de Vlam K, Covarrubias-Cobos JA, Mease PJ, Gladman DD, Chen L, Kudlacz E, Wu J, Cappelleri JC, Hendrikx T, Hsu MA (2019) Effect of tofacitinib on patient-reported outcomes in patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors in the phase III, randomised controlled trial: OPAL Beyond. RMD Open 5(1):e000808. https://doi.org/10.1136/rmdopen-2018-000808
Zhou HY, Guo B, Lufumpa E, Li XM, Chen LH, Meng X, Li BZ (2020) Comparative of the effectiveness and safety of biological agents, tofacitinib, and fecal microbiota transplantation in ulcerative colitis: systematic review and network meta-analysis. Immunol Invest 10(1080/08820139):1714650
Damiao A, de Azevedo MFC, Carlos AS, Wada MY, Silva TVM, Feitosa FC (2019) Conventional therapy for moderate to severe inflammatory bowel disease: a systematic literature review. World J Gastroenterol 25(9):1142–1157. https://doi.org/10.3748/wjg.v25.i9.1142
Gomez-Gomez A, Loza E, Rosario MP, Espinosa G, Morales J, Herreras JM, Munoz-Fernandez S, Cordero-Coma M (2017) Efficacy and safety of immunomodulatory drugs in patients with anterior uveitis: a systematic literature review. Medicine (Baltimore) 96(42):e8045. https://doi.org/10.1097/MD.0000000000008045
Espinosa G, Munoz-Fernandez S, Ruiz G, de Morales JM, Herreras JM, Cordero-Coma M (2017) Treatment recommendations for non-infectious anterior uveitis. Med Clin (Barc). https://doi.org/10.1016/j.medcli.2017.06.059
den Braanker H, Wervers K, Mus AMC, Bangoer PS, Davelaar N, Luime J, Tchetverikov I, Hazes JMW, Vis M, Lubberts E, Kok MR (2020) Achieving sustained minimal disease activity with methotrexate in early interleukin 23-driven early psoriatic arthritis. RMD Open. https://doi.org/10.1136/rmdopen-2020-001175
de Jong HMY, van Mens LJJ, Nurmohamed MT, Kok MR, van Kuijk AWR, Baeten DLP, van de Sande MGH (2019) Sustained remission with methotrexate monotherapy after 22-week induction treatment with TNF-alpha inhibitor and methotrexate in early psoriatic arthritis: an open-label extension of a randomized placebo-controlled trial. Arthritis Res Ther 21(1):208. https://doi.org/10.1186/s13075-019-1998-4
Coates LC, Moverley AR, McParland L, Brown S, Navarro-Coy N, O’Dwyer JL, Meads DM, Emery P, Conaghan PG, Helliwell PS (2015) Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 386(10012):2489–2498. https://doi.org/10.1016/S0140-6736(15)00347-5
Coates LC, Mahmood F, Freeston J, Emery P, Conaghan PG, Helliwell PS (2020) Long-term follow-up of patients in the TIght COntrol of inflammation in early Psoriatic Arthritis (TICOPA) trial. Rheumatology (Oxford) 59(4):807–810. https://doi.org/10.1093/rheumatology/kez369
Korotaeva TV, Loginova EY, Getiya TS, Nasonov EL (2018) Results of one-year treat-to-target strategy in early psoriatic arthritis: data of an open-label REMARCA study. Ter Arkh 90(5):22–29. https://doi.org/10.26442/terarkh201890522-29
Goulabchand R, Mouterde G, Barnetche T, Lukas C, Morel J, Combe B (2014) Effect of tumour necrosis factor blockers on radiographic progression of psoriatic arthritis: a systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis 73(2):414–419. https://doi.org/10.1136/annrheumdis-2012-202641
van der Heijde D, Gladman DD, FitzGerald O, Kavanaugh A, Graham D, Wang C, Fallon L (2019) Radiographic progression according to baseline C-reactive protein levels and other risk factors in psoriatic arthritis treated with tofacitinib or adalimumab. J Rheumatol 46(9):1089–1096. https://doi.org/10.3899/jrheum.180971
Behrens F, Cañete JD, Olivieri I, van Kuijk AW, McHugh N, Combe B (2015) Tumour necrosis factor inhibitor monotherapy vs combination with MTX in the treatment of PsA: a systematic review of the literature. Rheumatology (Oxford) 54(5):915–926. https://doi.org/10.1093/rheumatology/keu415
Aaltonen K, Heinonen A, Joensuu J, Parmanne P, Karjalainen A, Varjolahti-Lehtinen T, Uutela T, Puurtinen-Vilkki M, Arstila L, Blom M, Sokka T, Nordstrom D (2017) Effectiveness and drug survival of TNF-inhibitors in the treatment of psoriatic arthritis: a prospective cohort study. Semin Arthritis Rheum 46(6):732–739. https://doi.org/10.1016/j.semarthrit.2016.09.005
Zisapel M, Zisman D, Madar-Balakirski N, Arad U, Padova H, Matz H, Maman-Sarvagyl H, Kaufman I, Paran D, Feld J, Litinsky I, Wigler I, Caspi D, Elkayam O (2015) Prevalence of TNF-alpha blocker immunogenicity in psoriatic arthritis. J Rheumatol 42(1):73–78. https://doi.org/10.3899/jrheum.140685
European Medicines Agency EMA (2021) Xelyanz. Sumary of product. https://www.ema.europa.eu/en/medicines/human/EPAR/xeljanz#product-information-section
Haddad A, Li S, Thavaneswaran A, Cook RJ, Chandran V, Gladman DD (2016) The incidence and predictors of infection in psoriasis and psoriatic arthritis: results from longitudinal observational cohorts. J Rheumatol 43(2):362–366. https://doi.org/10.3899/jrheum.140067
Baumrin E, Van Voorhees A, Garg A, Feldman SR, Merola JF (2019) A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol 81(1):102–110. https://doi.org/10.1016/j.jaad.2019.03.017
Marra F, Lo E, Kalashnikov V, Richardson K (2016) Risk of herpes zoster in individuals on biologics, disease-modifying antirheumatic drugs, and/or corticosteroids for autoimmune diseases: a systematic review and meta-analysis. Open Forum Infect Dis. https://doi.org/10.1093/ofid/ofw205
Schreiber S, Colombel JF, Feagan BG, Reich K, Deodhar AA, McInnes IB, Porter B, Das Gupta A, Pricop L, Fox T (2019) Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis 78(4):473–479. https://doi.org/10.1136/annrheumdis-2018-214273
Genovese MC, Mysler E, Tomita T, Papp KA, Salvarani C, Schwartzman S, Gallo G, Patel H, Lisse JR, Kronbergs A, Leage SL, Adams DH, Xu W, Marzo-Ortega H, Lebwohl MG (2020) Safety of ixekizumab in adult patients with plaque psoriasis, psoriatic arthritis and axial spondyloarthritis: data from 21 clinical trials. Rheumatology (Oxford) 59(12):3834–3844. https://doi.org/10.1093/rheumatology/keaa189
Fiorentino D, Ho V, Lebwohl MG, Leite L, Hopkins L, Galindo C, Goyal K, Langholff W, Fakharzadeh S, Srivastava B, Langley RG (2017) Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2017.07.013
Kwan YH, Lim KK, Fong W, Goh H, Ng L, Haaland B, Phang JK, Low LL, Yeo JG, Huang F, Leung YY, Thumboo J, Ostbye T (2020) Risk of malignancies in patients with spondyloarthritis treated with biologics compared with those treated with non-biologics: a systematic review and meta-analysis. Ther Adv Musculoskelet Dis. https://doi.org/10.1177/1759720X20925696
Silva-Fernandez L, Perez-Vicente S, Martin-Martinez MA, Lopez-Gonzalez R, em ARIISG (2015) Variability in the prescription of non-biologic disease-modifying antirheumatic drugs for the treatment of spondyloarthritis in Spain. Semin Arthritis Rheum 44(6):633–640. https://doi.org/10.1016/j.semarthrit.2014.11.005
Canete JD, Tasende JAP, Laserna FJR, Castro SG, Queiro R (2020) The impact of comorbidity on patient-reported outcomes in psoriatic arthritis: a systematic literature review. Rheumatol Ther 7(2):237–257. https://doi.org/10.1007/s40744-020-00202-x
Acknowledgements
To the following rheumatologists for their participation in the Delphi process: José A. Pinto Tasende, Sara Alonso Castro, Sabela Fernández Aguado, Natalia Palmou Fontana, Olga Maiz Alonso, Carolina Álvarez Castro, Chamaida Plasencia, Xavier Juanola, Mireia Moreno, Cristina Maciá, Miguel Angel Abad, Ana Laiz, Vicenç Torrente-Segarra, Alba Erra, Miriam Almirall, Delia Reina, Jordi Gratacós, Sergio Rodríguez Montero, Eugenio de Miguel, Carlos Guillem, Esperanza Naredo, Pilar Susana del Rio, Ricardo Blanco. To Estibaliz Loza, from the institute of musculoskeletal health (Instituto de Salud Musculoesquelética, INMUSC), for her contribution in the methodological coordination and manuscript writing.
Funding
This work was supported by Pfizer. Pfizer has no participation in the steering committee, the formulation of the set recommendations nor in the Delphi process or manuscript writing. Estibaliz Loza, from the institute of musculoskeletal health (Instituto de Salud Musculoesquelética, INMUSC), which in turn was funded by Pfizer, and helped in methodological coordination and manuscript writing.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by all authors. The first draft of the manuscript was written by Rosario García-Vicuña and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All of the authors received honoraria from Pfizer for participation in this project. Francisco Rebollo and Susana Gómez work for Pfizer. Agustí Sellas has received honoraria as speaker and for consulting from Pfizer, Lilly Novartis, Janssen, Amgen. Julio Ramírez has participated at symposiums and/or advisory boards organised by Pfizer, Abbvie, MSD, UCB, Novartis, BMS, Lilly, Janssen, Amgen and Roche and his department has received research grants from Pfizer, Abbvie, Novartis and Janssen. Rubén Queiro has received honoraria as speaker, researcher and consultor from Janssen, Lilly, MSD, Abbvie, Pfizer, UCB, Celgene-Amgen, Novartis. Rosario García-Vicuña reports personal fees from Pfizer, during the conduct of the study; grants, personal fees and non-financial support from Abbvie, grants, personal fees and non-financial support from BMS, personal fees from Biogen, personal fees from Celltrion, grants, personal fees and non-financial support from Lilly, grants, personal fees and non-financial support from Novartis, grants, personal fees and non-financial support from MSD, personal fees and non-financial support from Pfizer, grants, personal fees and non-financial support from Sanofi, grants, personal fees and non-financial support from Sandoz, grants from Janssen, outside the submitted work. Estibaliz Loza has received research grants from Janssen, Lilly, MSD, Abbvie, Pfizer, UCB, Celgene-Amgen, Roche, Abbvie, BMS, Novartis. Noemí Garrido has received personal fees from Pfizer, Abbie, Lilly, Gedeon Richter, Novartis, Sanofi y Janssen. The rest of authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Vicuña, R., Garrido, N., Gómez, S. et al. Management of particular clinical situations in psoriatic arthritis: an expert’s recommendation document based on systematic literature review and extended Delphi process. Rheumatol Int 41, 1549–1565 (2021). https://doi.org/10.1007/s00296-021-04877-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-021-04877-5