Abstract
Synthesized PEO:I2 complex composite films with different I2 concentrations were deposited onto fused silica substrates using a dip-coating method. Incorporation of PEO films with I2 increases the electrical conductivity of the composite, reaching a maximum of 46 mS/cm for 7 wt% I2. The optical and optoelectronic properties of the complex composite films were studied using the transmittance and reflectance spectra in the UV-Vis region. The transmittance of PEO decreases with increasing I2 content. From this study, the optical bandgap energy decreases from 4.42 to 3.28 eV as I2 content increases from 0 to 7 wt%. In addition, the refractive index for PEO films are in the range of 1.66 and 2.00.1H NMR spectra of pure PEO film shows two major peaks at 3.224 ppm and 1.038 ppm, with different widths assigned to the mobile polymer chains in the amorphous phase, whereas the broad component is assigned to the more rigid molecules in the crystalline phase, respectively. By adding I2 to the PEO, both peaks (amorphous and crystal) are shifted to lower NMR frequencies indicating that I2 is acting as a Lewis acid, and PEO is acting as Lewis base. Hence, molecular iodine reacts favorably with PEO molecules through a charge transfer mechanism, and the formation of triiodide (\({\mathrm{I}}_{3}^{-}\)), the iodite (\({\mathrm{IO}}_{2}^{-}\)) anion, \({\mathrm{I}}_{2} \cdot \cdot \cdot \mathrm{ PEO}\) and \({\mathrm{I}}_{2}^{+} \cdot \cdot \cdot \mathrm{ PEO}\) complexes. PEO:I2 complex composite films are expected to be suitable for optical, electrical, and optoelectronic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyethylene oxide (PEO) is a crucial polymer electrolyte due to its low cost, high chemical and electrochemical stability, high stability in reduction processes, and good solubility of metal ions [1,2,3,4,5,6]. The importance of the PEO is for practical applications such as fuel cells, lithium-ion batteries, hybrid power supercapacitors [3, 7]. Incorporating inorganic ions into the polymer reduces transparency due to the absorption and scattering of electromagnetic photons from ionic agglomerates [8,9,10,11]. These complex composite films have tantalizing applications in advanced optoelectronic devices [12, 13].
The main reasons for choosing PEO as the host polymer in this study are the semi-crystalline nature [14], besides the low absorption of visible light [15, 16]. The electrical conductivity of the polymer increased by incorporation with electron donors or acceptors [17]. Iodine (I2) was chosen as the electron acceptor dopant in the polymers because of its significant influence on the optical, electrical and dielectric properties [18, 19]. According to our previous study [20], PEO:I2 is an effective complex composite because of the significant variations in ionic conductivity and dielectric properties.
Previous work [20] investigated the effect of iodine doping in PEO films on dielectric relaxation, X-ray photoelectron spectroscopy, and morphological properties. To further tune the optical, optoelectronic and electrical properties, such as refractive index, optical bandgap, and electrical conductivity, and to better understand the nature of the interactions in PEO, this study presents a composite of PEO polymer with different doping levels with inorganic filler I2. The effect of varying ionic concentrations of PEO:I2 complex composite films on optical and electrical properties is discussed. Furthermore, this study aims to understand the doping mechanism of PEO:I2 complex composite.
Experimental sections
Sample preparation
Polyethylene oxide (PEO) of MW 300.000 g/mol and Iodine (I2) of MW 253.8089 g/mol were obtained from Sigma-Aldrich; all solutions were prepared in absolute methanol (MeOH). A polymeric stock solution of PEO/MeOH was prepared by dissolving 1.0 g PEO in 100 mL MeOH by stirring for 5 h at 45 °C. The ionic stock solution of I2/MeOH was prepared by dissolving 0.1 g of I2 in 100 mL MeOH by stirring for 5 h at room temperature. The complex composite solutions with 0, 1, 3, 5, and 7 wt% of I2 were prepared by mixing 20 mL of PEO/MeOH with a calculated volume of I2/MeOH by ultrasonic water bath until the solution became a yellow homogeneous solution. PEO:I2 complex composite films were deposited on fused silica substrates by the dip-coating technique for 2 h. After that, PEO:I2 complex composite films were accomplished by drying the resulting films at room temperature for 24 h. A mathematical model proposed by Al Bataineh et al. was used to calculate the film thickness, which turned out to be around 500 nm [21].
Characterization
The doping mechanism for the PEO:I2 complex composite was investigated by analyzing the chemical structure using an FTIR Microscope HYPERION 3000 from Bruker and HN MAS NMR (600.13 MHz, Bruker AVIII-600 spectrometer). In addition, the electrical conductivity and pH measurements were also performed with SevenGo Duo SG23 conductivity-pH, and the absorption bands for the composite solution were examined with a UV–Vis spectrophotometer (Hitachi U-3900H) with a total internal integrating sphere. Thermal stability was investigated by thermogravimetric analysis (NETZSCH Premier Technologies, Exton, PA, USA).
Results and discussion
FTIR and NMR spectroscopies
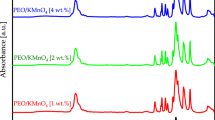
Figure 1 shows the chemical structure of pure PEO and PEO:I2 composites. The FTIR spectra show the typical vibrational bands of PEO, the vibrational band observed at 2880 cm−1 represents the stretching C–H bonds, 1466 cm−1 represents the scissoring CH2 bonds, 1340 cm−1 represents the wagging CH2 bonds, 1276 and 1235 cm−1 represent the twisting CH2 bonds, 955 and 840 cm−1 represent the rocking CH2 bonds, and 527 cm−1 represents the bending C–H bonds. Moreover, the triplet peak of the C–O–C stretching band at a maximum of 1088 cm−1 confirms the semicrystalline phase of PEO [22]. It is clear that the introduction of I2 into PEO has shifted the peak from 531 into 529 cm–1, indicating the appearance of C–I stretching vibrations and thus incorporating iodine with the polymer matrix. In addition, it can be seen that apparent changes in linewidth and peak position are observed with increasing I2 concentration. These changes represent the difference in the electronegativity between iodine and the polymer atoms, which can significantly affect the neighbouring group frequencies [18]. Furthermore, the change in intensity, linewidth and band position of the triplet peak of the C–O–C stretching band confirms the change in the degree of crystallinity [22].
1H NMR spectra of pure PEO film show two major peaks at 1.038 ppm and 3.224 ppm, with different widths (Fig. 2). Characteristically, the narrow component can be assigned, without ambiguity, to the mobile polymer chains in the amorphous phase, whereas the broad component is assigned to the more rigid molecules in the crystalline phase. Therefore, the peaks at 1.132 ppm and 3.174 ppm belong to the crystalline and the amorphous phase, respectively [23]. The amorphous phase peak has a symmetric shape at room temperature, while the NMR peak of the crystalline is still a broad asymmetric convoluted peak indicating that the crystalline phase is still in the rigid glassy form. Introducing I2 into the PEO matrix leads to increasing the linewidths of both peaks, but the changes are more pronounced in the peak associated with the amorphous phase. By adding I2 to the PEO, both peaks (amorphous and crystal) were shifted to the lower NMR frequencies indicating that the protons in PEO have a higher electron density. This means that I2 is acting as a Lewis acid, and PEO is acting as Lewis bases, hence, molecular iodine reacts favorably with PEO molecules through a charge transfer mechanism, to form charge transfer complexes (CTCs) as confirmed by electron spin resonance spectroscopy [24]. Additionally, the complexation also confirmed by the high-resolution XPS of the lower binding component confirmed the formation of triiodide (\({\mathrm{I}}_{3}^{-}\)) [25], the iodite (\({\mathrm{IO}}_{2}^{-}\)) anion, \({\mathrm{I}}_{2} \cdot \cdot \cdot \mathrm{ PEO}\) and \({\mathrm{I}}_{2}^{+} \cdot \cdot \cdot \mathrm{ PEO}\) complexes [26,27,28]. Additionally, XPS confirmed that \({\mathrm{I}}_{2} \cdot \cdot \cdot \mathrm{PEO}\) complex is shifted to a higher binding energy relative to the molecular \({\mathrm{I}}_{2}\), due to the partial positive charge on the \({\mathrm{I}}_{2}\) caused by the interaction with the more electronegative oxygen atoms in PEO [17].
pH measurement and electrical conductivity
Figure 3a shows the pH measurement results of the PEO:I2 complex composite solutions. pH values decrease as the I2 concentration increases in the composite, which translates into increased solution acidity with doping. Figure 3b shows the electrical conductivity for PEO:I2 complex composite solutions as a function of I2 concentration. The electrical conductivity of the PEO:I2 solution increased by four orders of magnitude with I2 doping, with a maximum of 46 mS/cm being achieved with 7 wt% of I2. Additionally, the conductivity of the PEO:I2 films also improves with increasing I2 concentration (Fig. 3c). The low electrical conductivity value associated with pure PEO can be attributed to the van der Waals interactions in polymer and the charge carriers hopping in the amorphous phase in this polymer [29]. Introducing I2 in the PEO matrix changes the gap-state occupation and, consequently, changes the electrical conductivity. Moreover, the electrical activity of iodine in the doped states can be affected by two factors: the first is related to the dangling bonds D0 and H-atoms of PEO, and the second is due to the creation of other dangling bond states D+ [30]. Also, iodine creates deep localized states in PEO, which leads to gap-state density changes by shifting the Fermi level and, consequently, increasing the electrical conductivity [31]. However, the electrical conductivity of the complex composite solutions is much higher than the electrical conductivity of the films, which can be attributed to the existence of diffusion of the iodine and the other factors that affect the electrical conductivity.
UV–Vis absorption spectroscopy
UV–Vis absorption spectroscopy was used to study the absorption bands of PEO:I2 complex composites (Fig. 4). Incorporation of PEO with I2 leads to a decrease in bandgap energy, shifting into the red region of the absorption bands and creating a new sub-band at 415.86 nm for 1 wt% I2 concentration, being the latter band red-shifted to 441.04 nm for 7 wt% I2 concentration. The absorbance spectra for PEO exhibit three transition bands with maxima at 216.69, 271.09, and 346.60 nm, with linewidths of 37.20, 70.66, and 191.62 nm, respectively. These three transitions can be assigned to polyenic domains in PEO [32]. According to molecular orbital theory [33], there are three possible electronic transitions for PEO: at 5.722 eV attributed to \(\pi \to {\pi }^{*}\) from unsaturated bonds [21], at 4.574 eV attributed to \(n\to {\pi }^{*}\) from carbonyl C–O bonds [34], and at 3.578 eV attributed to \(n\to {\sigma }^{*}\) from C–H bond.
The absorbance spectra of I2 in PEO illustrate four bands around 220, 290, 360, and 440 nm, which are assigned to the absorption of complex formation between PEO and I2, \({\mathrm{I}}^{-}\), \({\mathrm{I}}_{3}^{-}\), and I2 molecules, respectively [35, 36]. The absorption band between 440 and 500 nm represents the dissolving of I2 in PEO, identical to the spectra of I2 in alcohol and ethers [37]. The absorption shoulder typically appears at around 450 nm in thick PEO:I2 composite films, confirming that the iodine–iodide equilibrium is still present in PEO [38]. In conclusion, the dissolution of I2 in the PEO can be described as [39, 40]:
Thermogravimetric analysis
Thermogravimetric analysis (TGA) was used to study the thermal stability of PEO:I2 complex composites at temperatures up to 400 °C (Fig. 5a). The weight loss (%) of PEO:I2 complex composites decreases slightly in the range of 40–200 °C, due to water adsorption, intermolecular/intramolecular bonding, and chemical stability. Figure 5b shows the FTIR spectra for pure PEO and PEO:I2 (5 wt%) at different annealing temperatures. It is clear that at 200 °C there are no bond breaks. Between 200 and 300 °C, the weight loss (%) of pure PEO and PEO:I2 complex composites decreases again, which reflects the elimination of C–O stretch, C–H bend, and –CH2 stretch for pure PEO, and C–O stretch, C–H bend and –CH2 stretch in addition to C-I stretch for PEO:I2 composites, as shown in Fig. 5b. The weight loss (%) of pure PEO and PEO: I2 complex composites decreases sharply above 300 °C, attributed to the breaking of all bonds in the composites, as shown in Fig. 5b.
The maximum weight loss rate determined by DTG was observed at 325 °C for PEO, with this maximum rate shifting to higher temperatures as the I2 content increases in PEO. This indicates that the PEO:I2 complex composite has higher thermal stability compared to pure PEO. The increase in thermal stability of the PEO:I2 complex composite can be attributed to the difference in ionic radii in which I—ionic radii (1.33 Å) is lower than O—ionic radii (1.40 Å) [41]. Therefore, PEO: I2 complex composite films are expected to be stable at temperatures below 385 °C, where most optical, optoelectronic, and electrical applications can be achieved.
Optical and optoelectronic properties
The transmittance spectrum for PEO film increases to 93% as the wavelength increases from 250 to 400 nm and then reaches a plateau in the range of 400–700 nm (Fig. 6a). PEO:I2 complex composite films have lower transmittance than pure PEO film, albeit still having a high transmittance in the visible region, such as for PEO:I2 films with 7 wt% I2 with an average of 86%. The influence of I2 content with respect to PEO on the transmittance is the nonlinear effect. Broad dips in the transmittance spectra of the PEO:I2 films in the range of 350–450 nm are attributed to the X to B band transfer of I2. The absorption edge is continuously shifted into the red region with increasing I2 concentration, indicating a continuous reduction in bandgap energy due to the excitation of valence band electrons to the conduction band of I2 ions. The reflectance spectra (Fig. 6b) show the opposite curve progression with the transmittance spectra. The reflectance of PEO film decreases from 12.0 to 6.2% as the wavelength increases from 350 to 700 nm. Adding I2 to the PEO film increases reflectance values in the visible region.
a Transmittance, b reflectance, c extinction coefficient, and d refractive index spectra of PEO:I2 films for different I2 concentrations, e Transmittance spectra of PEO: I2 films for different I2 concentrations; experimental (colored circles) and model calculated (solid red lines). f Band-gap energy (\({E}_{\mathrm{g}}\)) and Urbach energy of PEO:I2 films as a function of I2 concentrations (colour figure online)
The extinction coefficient (k) is related to the absorption coefficient according to the equation \(k=\alpha \lambda /4\pi\) [42]. Figure 6c shows the \(k\)-spectra for PEO:I2 complex composite films. \(k\)-values decrease significantly below the absorption edge into the visible range, indicating that photons pass through the film in this range. As I2 content increases in the PEO films, the light energy loss also increases due to scattering and absorption [43]. The refractive index, \(n\), for the PEO:I2 complex composite films was deduced from the following equation \(n=\left(1+R/1-R\right)+\sqrt{\left(4R/{\left(1-R\right)}^{2}\right)-{k}^{2}}\) (Fig. 6d) [42]. The n-values for a pure PEO film decrease from 2.00 to 1.66 as the incident photon wavelength increases from 250 to 700 nm. Furthermore, increasing the I2 concentration in PEO films results in a concomitant increase in the refractive index, attributed to the iodine ions condensation into polyiodide clusters in the PEO films [44]. The wide drop in the refractive index spectra in the range of 250–350 nm for pure PEO film and 300–450 nm for PEO:I2 films can be attributed to the resonance effect between the incident photons and the electrons in the films [45].
A mathematical model developed by Al Bataineh et al. was used to investigate the bandgap energy (\({E}_{\mathrm{g}}\)) for PEO:I2 complex composite films according to the following relationship [21]:
where \(hc = 1239.84\; {\text{eV}}\;{\text{nm}}\), and \(A, B, C\) are fitting constants. Figure 6e shows the model fit of transmission values for pure PEO and PEO:I2 complex composite films. Figure 6f shows the bandgap energy variation obtained from this model. The bandgap energy of pure PEO film is 4.42 eV. As I2 content increased in the PEO:I2 films the band-gap decreases to a minimum of 3.28 eV for 7 wt% of I2. The interaction between PEO and I2 is an oxidative interaction, which means that the radical cation in the PEO is formed with iodine anion and consequently forms polarons and decreases the bandgap energy [20].
Urbach energy, \({E}_{\mathrm{U}},\) describes the disorder degree in the films by an empirical rule based on the following equation \(\alpha ={\alpha }_{0}\mathrm{exp}\left(hv/{E}_{\mathrm{U}}\right)\) [46]. The \({E}_{\mathrm{U}}\) values can be determined by plotting \(\mathrm{ln}(\alpha )\) as a function of \(hv\). Figure 6f shows the Urbach energy variation for all PEO:I2 films as a function of I2 concentration. The Urbach energy for pure PEO film is \(177.2\;{\text{meV}}\). This parameter increases with I2 content up to a maximum of \(225.3\;{\text{meV}}\) for 7 wt% of I2. This trend indicates an increase in the disorder of the films in addition to the increase in organic–inorganic interaction [47].
To understand the influence of I2 doping on the optical and optoelectronic properties of the complex composite films, models developed by Wemple–DiDomenico (WDD) [48, 49], Sellmeier [50], and Spitzer-Fan [51] were applied using the following equations, respectively,
Table 1 shows essential optical and optoelectronic parameters for pure PEO and PEO:I2 complex composite films for different I2 concentrations. Effective single oscillator energy (\({E}_{0}\)) and dispersive energy (\({E}_{\mathrm{d}}\)) (Eq. 1) is used for the design of dispersive and optical communication devices. Increasing I2 in PEO:I2 films leads to a decrease in \({E}_{0}\) and conversely an increase in \({E}_{\mathrm{d}}\), resulting from decreasing binding energy between the film elements [52]. Average oscillator wavelength (\({\lambda }_{0}\)) values increase from 197.13 to 198.53 nm, and oscillator length strength (\({S}_{0}\)) values increase from \(3.975\times {10}^{-5}\) to \(4.359\times {10}^{-5}\) as I2 content increases in PEO to 7 wt%. Consequently, the increase in \({\lambda }_{0}\) and \({S}_{0}\) is due to the decline in surface morphology of films with introducing I2 in PEO films. The high-frequency dielectric constant (\({\varepsilon }_{\infty }\)) for PEO film is 3.410. This value increases to 3.949 for 7 wt% of I2 in PEO. This also increases the charge carriers concentration from \(6.688\times {10}^{26}\) to \(7.029\times {10}^{26}\) atoms/\({\mathrm{m}}^{3}\) with the same increase in doping.
Conclusions
The doping mechanism for PEO:I2 was studied using FTIR spectroscopy, pH, electrical conductivity, UV–Vis absorbance spectroscopy, and TGA. The changes in peak position and linewidth of the absorption spectra are due to the incorporation of PEO and I2. In addition, introducing I2 into PEO leads to a shift of the peak at 535 cm–1, due to increased C–I stretching vibrations. Electrical conductivity increases concomitantly with increasing I2 doping in PEO films, while pH values decrease due to increasing solution acidity.
TGA with FTIR analysis at different annealing temperatures showed that the weight loss of pure PEO and PEO:I2 complex composites decreases slightly from 40 to 200 °C, attributed to the adsorbed water, intermolecular/intramolecular bonding and chemical stability. In addition, between 200 and 300 °C, the weight loss of pure PEO and PEO:I2 complex composites also decreases, due to the elimination of C–O stretch, C–H bend, and –CH2 stretch for pure PEO, and C–O stretch, C–H bend and –CH2 stretch in addition to C-I stretch. Above 300 °C, the weight loss of pure PEO and PEO:I2 complex composites decreases sharply, attributed to bond breaking the film composites.
The transmittance of the PEO film decreases with increasing I2 content, while the optical band gap energy decreases from 4.42 to 3.28 eV as I2 content increases from 0 to 7 wt%. In addition, the refractive index for PEO film has values ranging between 1.66 and 2.00 as the wavelength decreases from 700 to 350, and the average values of the refractive index increase as the concentration of I2 is increased up to 7 wt%.
The 1H NMR peak appearing at 1.038 ppm can be assigned to the mobile polymer chains in the amorphous phase while the peak at 3.224 ppm is assigned to the more rigid molecules in the crystalline phase which was still in the rigid glassy form at room temperature. By adding I2 to the PEO, both peaks (amorphous and crystal) were shifted to the lower NMR frequencies indicating that I2 is acting as a Lewis acid, and PEO is acting as Lewis bases and the formation of triiodide (\({\mathrm{I}}_{3}^{-}\)), the iodite (\({\mathrm{IO}}_{2}^{-}\)) anion, \({\mathrm{I}}_{2} \cdot \cdot \cdot \mathrm{ PEO}\) and \({\mathrm{I}}_{2}^{+} \cdot \cdot \cdot \mathrm{ PEO}\) complexes.
References
Bresser D, Lyonnard S, Iojoiu C, Picard L, Passerini S (2019) Decoupling segmental relaxation and ionic conductivity for lithium-ion polymer electrolytes. Mol Syst Des Eng 4:779
Scrosati B, Vincent CA (2000) Polymer electrolytes: the key to lithium polymer batteries. Mrs Bull 25(3):28–30
Xue Z, He D, Xie X (2015) Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J Mater Chem A 3(38):19218–19253
Mindemark J, Lacey MJ, Bowden T, Brandell D (2018) Beyond PEO—alternative host materials for Li+-conducting solid polymer electrolytes. Prog Polym Sci 81:114–143
Lin D, Yuen PY, Liu Y, Liu W, Liu N, Dauskardt RH, Cui Y (2018) A silica-aerogel-reinforced composite polymer electrolyte with high ionic conductivity and high modulus. Adv Mater 30(32):1802661
Aldalur I, Martinez-Ibañez M, Piszcz M, Rodriguez-Martinez LM, Zhang H, Armand M (2018) Lowering the operational temperature of all-solid-state lithium polymer cell with highly conductive and interfacially robust solid polymer electrolytes. J Power Sources 383:144–149
Karmakar A, Ghosh A (2012) Dielectric permittivity and electric modulus of polyethylene oxide (PEO)–LiClO4 composite electrolytes. Curr Appl Phys 12(2):539–543
Liu J-G, Ueda M (2009) High refractive index polymers: fundamental research and practical applications. J Mater Chem 19(47):8907–8919
Hassan M, Reddy KR, Haque E, Minett AI, Gomes VG (2013) High-yield aqueous phase exfoliation of graphene for facile nanocomposite synthesis via emulsion polymerization. J Colloid Interface Sci 410:43–51
Reddy KR, Sin BC, Ryu KS, Kim J-C, Chung H, Lee Y (2009) Conducting polymer functionalized multi-walled carbon nanotubes with noble metal nanoparticles: synthesis, morphological characteristics and electrical properties. Synth Met 159(7–8):595–603
Tao P, Li Y, Rungta A, Viswanath A, Gao J, Benicewicz BC, Siegel RW, Schadler LS (2011) TiO2 nanocomposites with high refractive index and transparency. J Mater Chem 21(46):18623–18629
Mosley DW, Auld K, Conner D, Gregory J, Liu X-Q, Pedicini A, Thorsen D, Wills M, Khanarian G, Simon ES (2008) High performance encapsulants for ultra high-brightness LEDs. In: Light-emitting diodes: research, manufacturing, and applications XII, vol 6910. International Society for Optics and Photonics, p 691017
Nakamura T, Fujii H, Juni N, Tsutsumi N (2006) Enhanced coupling of light from organic electroluminescent device using diffusive particle dispersed high refractive index resin substrate. Opt Rev 13(2):104–110
Migdadi A, Ahmad AA, Alsaad AM, Al-Bataineh QM, Telfah A (2022) Electrical and thermal characterizations of synthesized composite films based on polyethylene oxide (PEO) doped by aluminium chloride (AlCl3). Polym Bull. https://doi.org/10.1007/s00289-022-04329-5
Alsaad A, Al-Bataineh QM, Qattan I, Ahmad AA, Ababneh A, Albataineh Z, Aljarrah IA, Telfah A (2020) Measurement and ab initio Investigation of structural, electronic, optical, and mechanical properties of sputtered aluminum nitride thin films. Front Phys 8:115
Ohring M (2001) Materials science of thin films. Elsevier
Telfah A, Al-Akhras M-A, Al-Izzy KA, Ahmad AA, Ababneh R, Ahmad MJA, Tavares CJ, Hergenröder R (2022) Dielectric relaxation, XPS and structural studies of polyethylene oxide/iodine complex composite films. Polym Bull 79(6):3759–3778
Bazaka K, Jacob MV (2017) Effects of iodine doping on optoelectronic and chemical properties of polyterpenol thin films. Nanomaterials 7(1):11
Telfah M, Ahmad A, Alsaad A, Al-Bataineh QM, Telfah A (2022) Doping mechanism and optical properties of as-prepared polyvinyl chloride (PVC) doped by iodine thin films. Polym Bull. https://doi.org/10.1007/s00289-022-04082-9
Telfah A, Al-Akhras M-A, Al-Izzy KA, Ahmad AA, Ababneh R, Ahmad MJA, Tavares CJ, Hergenröder R (2021) Dielectric relaxation, XPS and structural studies of polyethylene oxide/iodine complex composite films. Polym Bull 79:1–20
Al-Bataineh QM, Alsaad A, Ahmad A, Telfah A (2020) A novel optical model of the experimental transmission spectra of nanocomposite PVC-PS hybrid thin films doped with silica nanoparticles. Heliyon 6(6):e04177
Noor S, Ahmad A, Talib I, Rahman MY (2010) Morphology, chemical interaction, and conductivity of a PEO-ENR50 based on solid polymer electrolyte. Ionics 16(2):161–170
Johansson A, Tegenfeldt J (1992) NMR study of crystalline and amorphous poly(ethylene oxide). Macromolecules 25(18):4712–4715
Danyluk S, Schneider W (1962) The electron spin resonance of non-alternant aromatic hydrocarbon–iodine complexes. Can J Chem 40(9):1884–1885
Kumar GR, Savariraj AD, Karthick S, Selvam S, Balamuralitharan B, Kim H-J, Viswanathan KK, Vijaykumar M, Prabakar K (2016) Phase transition kinetics and surface binding states of methylammonium lead iodide perovskite. Phys Chem Chem Phys 18(10):7284–7292
Zhang J, Song T, Zhang Z, Ding K, Huang F, Sun B (2015) Layered ultrathin PbI2 single crystals for high sensitivity flexible photodetectors. J Mater Chem C 3(17):4402–4406
Salaneck W, Thomas H, Bigelow R, Duke C, Plummer E, Heeger A, MacDiarmid A (1980) Photoelectron spectroscopy of iodine-doped polyacetylene. J Chem Phys 72(6):3674–3678
Sherwood P (1976) NIST X-ray photoelectron spectroscopy database. J Chem Soc Faraday Trans II 72:1806
Masoud EM, El-Bellihi A-A, Bayoumy W, Mousa M (2013) Organic–inorganic composite polymer electrolyte based on PEO–LiClO4 and nano-Al2O3 filler for lithium polymer batteries: Dielectric and transport properties. J Alloys Compd 575:223–228
Tyczkowski J (2004) Electrical and optical properties of plasma polymers. Plasma Polymer Films. Imperial College Press, pp 143–216
Arkhipov V, Heremans P, Emelianova E, Adriaenssens G, Bässler H (2004) Charge carrier mobility in doped disordered organic semiconductors. J Non-Cryst Solids 338:603–606
El-Ghamaz N, Ghaly H (2016) Effect of chemical and physical doping with iodine on the optical and dielectric properties of poly(vinyl chloride). Chem Phys Lett 648:66–74
Fleming I (2011) Molecular orbitals and organic chemical reactions. Wiley
Al-Bataineh QM, Telfah M, Ahmad AA, Alsaad AM, Qattan IA, Baaziz H, Charifi Z, Telfah A (2020) Synthesis, crystallography, microstructure, crystal defects, optical and optoelectronic properties of ZnO:CeO2 mixed oxide thin films. Photonics 7(4):112
Zidan HM, Abdelrazek EM, Abdelghany AM, Tarabiah AE (2019) Characterization and some physical studies of PVA/PVP filled with MWCNTs. J Mater Res Technol 8(1):904–913
Ryu SH, Cho H-B, Kim S, Kwon Y-T, Lee J, Park K-R, Choa Y-H (2018) The effect of polymer particle size on three-dimensional percolation in core-shell networks of PMMA/MWCNTs nanocomposites: properties and mathematical percolation model. Compos Sci Technol 165:1–8
Kumar A, Kumar V, Kumar M, Awasthi K (2018) Synthesis and characterization of hybrid PANI/MWCNT nanocomposites for EMI applications. Polym Compos 39(11):3858–3868
Wu G, Jia Z, Zhou X, Nie G, Lv H (2020) Interlayer controllable of hierarchical MWCNTs@C@FexOy cross-linked composite with wideband electromagnetic absorption performance. Compos A Appl Sci Manuf 128:105687
Kim K-I, Kim D-A, Patel KD, Shin US, Kim H-W, Lee J-H, Lee H-H (2019) Carbon nanotube incorporation in PMMA to prevent microbial adhesion. Sci Rep 9(1):1–11
Karlsen EM, Spanget-Larsen J (2009) FTIR investigation of the reaction between pyridine and iodine in a polyethylene host. Formation of N-iodopyridinium polyiodide. Chem Phys Lett 473(4–6):227–232
Shariati A, Omidkhah M, Pedram MZ (2012) New permeation models for nanocomposite polymeric membranes filled with nonporous particles. Chem Eng Res Des 90(4):563–575
Ahmad A, Alsaad A, Al-Bataineh Q, Al-Naafa M (2018) Optical and structural investigations of dip-synthesized boron-doped ZnO-seeded platforms for ZnO nanostructures. Appl Phys A 124(6):458
Mott NF, Davis EA (2012) Electronic processes in non-crystalline materials. Oxford University Press
Oubaha M, Elmaghrum S, Copperwhite R, Corcoran B, McDonagh C, Gorin A (2012) Optical properties of high refractive index thin films processed at low-temperature. Opt Mater 34(8):1366–1370
Alsaad A, Marin CM, Alaqtash N, Chao H-W, Chang T-H, Cheung CL, Ahmad A, Qattan I, Sabirianov RF (2018) Crystallographic, vibrational modes and optical properties data of α-DIPAB crystal. Data Brief 16:667–684
Aziz SB, Abdullah OG, Brza M, Azawy AK, Tahir DA (2019) Effect of carbon nano-dots (CNDs) on structural and optical properties of PMMA polymer composite. Results Phys 15:102776
Chamroukhi H, Hamed ZB, Telfah A, Bassou M, Zeinert A, Hergenröder R, Bouchriha H (2018) Optical and structural properties enhancement of hybrid nanocomposites thin films based on polyaniline doped with Zinc Oxide embedded in bimodal mesoporous silica (ZnO@SiOX) nanoparticles. Opt Mater 84:703–713
Sutcliffe BT, Wilson S (2003) Potential energy curves and surfaces. Handbook of Molecular Physics and Quantum Chemistry, pp 574–587
Fu DW, Zhang W, Cai HL, Ge JZ, Zhang Y, Xiong RG (2011) Diisopropylammonium chloride: a ferroelectric organic salt with a high phase transition temperature and practical utilization level of spontaneous polarization. Adv Mater 23(47):5658–5662
Jundale D, Pawar S, Chougule M, Godse P, Patil S, Raut B, Sen S, Patil V (2011) Nanocrystalline CuO thin films for H2S monitoring: microstructural and optoelectronic characterization. J Sens Technol 1:36–46
Fasasi A, Osagie E, Pelemo D, Obiajunwa E, Ajenifuja E, Ajao J, Osinkolu G, Makinde WO, Adeoye AE (2018) Effect of precursor solvents on the optical properties of copper oxide thin films deposited using spray pyrolysis for optoelectronic applications. Am J Mater Synth Process 3:12–22
Hassanien AS, Akl AA (2015) Influence of composition on optical and dispersion parameters of thermally evaporated non-crystalline Cd50S50−xSex thin films. J Alloys Compd 648:280–290
Acknowledgements
The scientific support by the Ministerium für Innovation, Wissenschaft und Forschung des Landes Nordrhein-Westfalen, the Senatsverwaltung für Wirtschaft, Technologie und Forschung des Landes Berlin, and the Bundesministerium für Bildung und Forschung is gratefully acknowledged. This study was supported by the German Ministry of Research and Education (ERK-Casting, 16GW0262K), and the Drug Discovery Hub Dortmund (DDHD). The authors would like to acknowledge Jordan University of Science and Technology. Our thanks also to Prof. Mohammad-Ali H. Al-Akhras for helping our members to use the biomedical laboratory.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Telfah, A., Al-Bataineh, Q.M., Tolstik, E. et al. Optical, electrical and chemical properties of PEO:I2 complex composite films. Polym. Bull. 80, 9611–9625 (2023). https://doi.org/10.1007/s00289-022-04508-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04508-4