Abstract
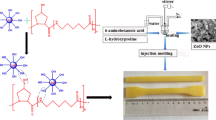
In this study, nanocomposites of acrylamide (AAm) and 2-hydroxyethyl methacrylate (HEMA)-based hydrogel containing ZnO nanoparticles in different ratios by mass (1, 3, and 5%) were synthesized using in situ polymerization/hydrothermal method. It was determined that the hydrogel contained 60% HEMA in the copolymer composition calculated with the help of 1H-NMR. Characterization of materials was carried out by SEM, BET, FTIR, UV–Vis, TGA, and XRD techniques. The Ea and Tg value and thermal stability of the pure poly(AAm-co-HEMA) hydrogel were increased by adding ZnO. The water absorption capacity of nanocomposites was determined by swelling experiments. As the amount of ZnO in the composites increased, the degree of swelling decreased. Biological activities of poly(AAm-co-HEMA) and nanocomposites against gram-positive (S. aureus) and gram-negative (E. coli) bacteria increased as the amount of ZnO increased. Finally, The dielectric properties of the poly(AAm-co-HEMA) and nanocomposites were investigated as a function of frequency at 25 °C temperature in the frequency range of 1–200 kHz.

















Similar content being viewed by others
References
Date P, Tanwar A, Ladage P et al (2020) Carbon dots-incorporated pH-responsive agarose-PVA hydrogel nanocomposites for the controlled release of norfloxacin drug. Polym Bull 77:5323–5344
Hyon SH, Cha WI, Ikada Y (1989) Preparation of transparent poly(vinyl alcohol) hydrogel. Polym Bull 22:119–122
Bush JR, Liang H, Dickinson M, Botchwey EA (2016) Xylan hemicellulose improves chitosan hydrogel for bone tissue regeneration. Polym Adv Technol 27(8):1050–1055
Kashyap N, Kumar N, Kumar M (2005) Hydrogels for pharmaceutical and biomedical applications. Crit Rev Ther Drug Carr Syst 22:107–149
Adimule V, Yallur BC, Bhowmik D, Gowda AHJ (2022) Dielectric properties of P3BT doped ZrY2O3/CoZrY2O3 nanostructures for low cost optoelectronics applications. Trans Electr Electron Mater 23:288–303
Adimule V, Yallur BC, Keri R (2022) Studies on synthesis, characterization of SMX ZNO:coo nanocomposites and its effect on photo catalytic degradation of textile dyes Top Catal https://doi.org/10.1007/s11244-022-01574-w
Pai MM, Batakurki SR, Yallur BC et al (2022) Green synthesis of chitosan supported magnetic palladium nanoparticles using epiphyllum oxypetalum leaf extract (Pd-CsEo/Fe3O4 NPs) as hybrid nanocatalyst for suzuki-miyaura coupling of thiophene. Top Catal. https://doi.org/10.1007/s11244-022-01576-8
Keri RS, Adimule V, Kendrekar P et al (2022) The nano-based catalyst for the synthesis of benzimidazoles. Top Catal. https://doi.org/10.1007/s11244-022-01562-0
Shaikh NM, Adimule V, Bagihalli GB et al (2022) A novel mixed Ag–Pd nanoparticles supported on SBA silica through [DMAP-TMSP-DABCO]OH basic ionic liquid for suzuki coupling reaction. Top Catal. https://doi.org/10.1007/s11244-022-01586-6
Shaikh NM, Sawant AD, Bagihalli GB et al (2022) Highly active mixed Au–Pd nanoparticles supported on RHA silica through immobilised ionic liquid for suzuki coupling reaction. Top Catal. https://doi.org/10.1007/s11244-021-01547-5
Adimule VM, Nandi SS, Kerur SS et al (2022) Recent advances in the one-pot synthesis of coumarin derivatives from different starting materials using nanoparticles: a review. Top Catal. https://doi.org/10.1007/s11244-022-01571-z
Adimule V, Kerur SS, Chinnam S et al (2022) Guar gum and its nanocomposites as prospective materials for miscellaneous applications: a short review. Top Catal. https://doi.org/10.1007/s11244-022-01587-5
Singh B, Chauhan N, Kumar S, Bala R (2008) Psyllium and copolymers of 2-hydroxylethylmethacrylate and acrylamide-based novel devices for the use in colon specific antibiotic drug delivery. Int J Pharm 352(1–2):74–80
Nizam El-Din HM, El-Naggar AWM (2005) Synthesis and characterization of hydroxyethyl methacrylate/acrylamide responsive hydrogels. J App Polym Sci 95(5):1105–1115
Tanan W, Saengsuwan S (2014) Microwave assisted synthesis of poly (acrylamide-CO-2-hydroxyethyl methacrylate)/poly(vinyl alcohol) semi-IPN hydrogel. Energy Procedia 56:386–393
Rapado M, Peniche C (2015) Synthesis and characterization of pH and temperature responsive poly(2-hydroxyethyl methacrylate-co-acrylamide) hydrogels. Polím 25(6):547–555
Alam S, Khan L, Shah LA et al (2020) Synthesis of copolymeric hydrogels of acrylamide and 2-(hydroxyethyl methacrylate) and its use for the adsorption of basic blue 3 dye. Z Pys Chem 235(6):707–721
Shukla S, Bajpai AK (2013) Plaster of paris-reinforced nanocomposites of poly (2-hydroxyethyl methacrylate-co-acrylamide) as alternative orthopedic material. Polym Plast Technol Eng 52(2):133–140
Bharti DB, Bharati AV (2016) Synthesis of ZnO nanoparticles using a hydrothermal method and a study its optical activity. Luminescenc 32(3):317–320
Maryanti E, Damayanti D, Gustian I, Yudha SS (2014) Synthesis of ZnO nanoparticles by hydrothermal method in aqueous rinds extracts of Sapindus rarak DC. Mater Lett 118:96–98
Ritger PL, Peppas NA (1987) A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs spheres cylinders or discs J Controll Release 5 (1): 23–36
Xiong G, Pal U, Serrano J et al (2006) Photoluminesence and FTIR study of ZnO nanoparticles: the impurity and defect perspective. Phy Status Solidi 3(10):3577–3581
Znaidi L, Soler Illia GJAA, Benyahia S et al (2003) Oriented ZnO thin films synthesis by sol-gel process for laser application. Thin Solid Films 428:257–262
Anzlovar A, Kogej K, Crnjak Orel Z, Zigon M (2011) Polyol mediated nano size zinc oxide and nanocomposites with poly(methyl methacrylate). Express Polym Lett 5:604–619
Sinha A, Sharma BP (2002) Preparation of copper powder by glycerol process. Mat Res Bull 37:407–416
Heller RB, McGannon J, Weber AH (1950) Precision determination of the lattice constants of zinc oxide. J App Phys 21:1283–1284
Fujihara S, Naito H, Kimura T (2001) Visible photoluminescence of ZnO nanoparticles dispersed in highly transparent MgF thin-films via solgel process. Thin Solid Films 389:2227–2732
Nyffenegger RM, Craft B, Shaaban M et al (1998) Hybrid electrochemical/chemical synthesis of zinc oxide nanoparticles and optically intrinsic thin films. Chem Mater 10:1120–1129
Wang Y, Herron N (1991) Nanometer-sized semiconductor clusters: materials synthesis, quantum size effects, and photophysical properties. J Phys Chem 95:525–532
Balen R, Costa WV, Andrade JL et al (2016) Structural, thermal, optical properties and cytotoxicity of PMMA/ZnO fibers and films: potential application in tissue engineering. Appl Surf Sci 385:257–276
Paramo JA, Strzhemechny YM, Anžlovar A et al (2010) Enhanced room temperature excitonic luminescence in ZnO/polymethyl methacrylate nanocomposites prepared by bulk polymerization. J Appl Phys 108:023517
Zhang L, Li F, Chen Y, Wang X (2011) Synthesis of transparent ZnO/PMMA nanocomposite films through free-radical copolymerization of asymmetric zinc methacrylate acetate and in-situ thermal decomposition. J Lumin 131:1701–1706
Khan M, Chen M, Wei C (2014) Synthesis at the nanoscale of ZnO into poly(methyl methacrylate) and its characterization. Appl Phys A 117:1085–1093
Soykan C, Erol I, Kirbag S (2003) Synthesis and characterization of poly(1,3-thiazol-2-yl-carbomoyl) methyl methacrylate: its metal complexes and antimicrobial activity studies. J Appl Polym Sci 90:3244–3251
Achilias DS (2007) A review of modelling of diffusion controlled polymerization reactions. Macromol Theory Simul 16:319–347
Flynn JH, Wall LA (1966) A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B: Polym Lett 4(5):323–328
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29(11):1702–1706
Nunez L, Fraga F, Nunez MR, Villanueva M (2000) Thermogravimetric study of the decomposition process of the system BADGE (n=0)/1,2 DCH. Polym 41(12):4635–4641
Kurt A (2009) Thermal decomposition kinetics of poly(nButMA-b-St) diblock copolymer synthesized by ATRP. J Appl Polym Sci 114(1):624–629
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201:68–69
Kurt A, Avcı HI, Koca M (2018) Synthesis and characterization of a novel isocoumarin derived polymer and its thermal decomposition kinetics. Maced J Chem Chem Eng 37:173–184
Işik B (2000) Swelling behavior of acrylamide-2-hydroxyethyl methacrylate hydrogels. Turk J Chem 24(2):147–156
Peppas NA, Bures P, Leobandung W, Ichikawa H (2000) Hyrdogels in pharmaceutical formulations. Eur J Pharm Biopharm 50:27–46
Ganji F, Farahani SV, Farahani EV (2010) Theoretical description of hydrogel swelling: a review. Iran Polym J 19:375–398
Shrivastava S, Bera T, Roy A et al (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18(22):225103
Saliani M, Jalal J, Kafshdare EG (2015) Effects of pH and temperature on antibacterial activity of zinc oxide nanofluid against e. coliO157:H7 and Staphylococcus aureus Jundishapur J Microbiol 8(2): e17115
Jin SE, Jin HE (2021) Antimicrobial activity of zinc oxide nano/microparticles and their combinations against pathogenic microorganisms for biomedical applications: from physicochemical characteristics to pharmacological aspects. Nanomater 11(2):263
Chappard D, Filmon R, Grizon F, Basle MF (2002) Effects of negatively charged groups (carboxymethyl) on the calcification of poly(2-hydroxyethyl methacrylate). Biomater 23:3053–3059
Ma X, Wang H, Jin S, Wu Y, Liang XJ (2012) Construction of paclitaxel-loaded poly(2-hydroxyethylmethacrylate)-gpoly(lactide)-1,2-dipalmitoyl-sn-glycero-3-hosphoethanolamine copolymer nanoparticle delivery system and evaluation of its anticancer activity. Int J Nanomed 7:1313–1328
Zhou W, Li H, Xia C et al (2009) The synthesis and biological evaluation of some caffeic acid amide derivatives: E-2-Cyano-(3-substituted phenyl)acrylamides. Bioorg Med Chem Lett 19(7):1861–1865
Nitsche C, Steuer C, Klein CD (2011) Arylcyanoacrylamides as inhibitors of the dengue and west nile virus proteases. Bioorg Med Chem J 19(24):7318–7337
Sato I, Morihira K, Inami H et al (2009) Synthesis, biological evaluation, and metabolic stability of acrylamide derivatives as novel CCR3 antagonists. Bioorg Med Chem 17(16):5989–6002
Zhou N, Zeller W, Zhang J (2009) Synthesis, biological evaluation and metabolic stability of potent and selective EP3 receptor antagonist. Bioorganic Med Chem Lett 19(5):1528–1531
Onda K, Shiraki R, Yonetoku Y et al (2008) Synthesis and pharmacological evaluation of bis-3-(3,4-dichlorophenyl) acrylamide derivatives as glycogen phosphorylase inhibitors. Bioorg Med Chem 16(18):8627–8634
Fu J, Cheng K, Zhang Z et al (2010) Synthesis, structure and structure–activity relationship analysis of caffeic acid amides as potential antimicrobials. Eur J Med Chem 45(6):2638–2643
Xu L (2008) Characterization and biological activities of novel acrylamide compounds. Chem Res Chin Univ 24(5):575–578
Zheng W, Wong SC (2003) Electrical conductivity and dielectric properties of PMMA/expanded graphite composites. Compos Sci Technol 63:225–235
Bal KK, Kothari VK (2009) Measurement of dielectric properties of textile materials and their applications. Ind J Fıbre Tex Res 191–9
Beladakere NN, Misra SCK, Ram MK, Rout DK, Gupta R, Malhotra BD et al (1992) Interfacial polarization in semiconducting polypyrrole thin films. J Phys: Condens Matter 4:5747–5756
Raja V, Sharma AK, Rao N (2004) Impedance spectroscopic and dielectric analysis of PMMA-co-P4VPNO polymer films. Mate Lett 58:3242–3247
Acknowledgements
This study has been supported by the Afyon Kocatepe University Scientific Research Projects Coordination Unit. The Project Number is 21-FENED-05.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erol, I., Aksu, M. & Gürler, Z. Preparation of poly(AAm-co-HEMA)/ZnO nanocomposites via in situ polymerization/hydrothermal method and determination of their properties. Polym. Bull. 80, 5675–5703 (2023). https://doi.org/10.1007/s00289-022-04343-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04343-7