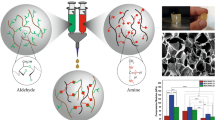
Abstract
Along with providing an environment for cell attachment and proliferation, a tissue engineering scaffolds should possess physical and mechanical properties that would fit the target tissue. The present study aimed to manipulate physico-mechanical properties of polyacrylamide/gelatin hydrogels using response surface method-central composite design (RSM-CCD) to reach a scaffold with defined properties. On this demand, mixtures of gelatin and acrylamide (AAm) monomer were used to prepare semi-interpenetrating hydrogels by free radical polymerization of AAm. Selected variables for statistical modeling were chosen to be weight ratios of monomer/crosslinker, monomer/gelatin, and monomer/initiator. The desired responses were compressive modulus, compressive strength, and swelling. Results showed that desired responses could be tailored by varying these parameters with the highest impact for monomer/crosslinker ratio. The swelling ratio of hydrogels was in the range of 947–1654%, while the modulus varied between 5 and 35 kPa. The cyclic compressive test showed the durability of hydrogels under cyclic loadings. Finally, the results of cell attachment and cytocompatibility analyses indicated that the hydrogels were completely biocompatible and enhanced cell attachment. Thus, these hydrogels could potentially be used as tissue engineering scaffolds for load-bearing organs, including muscle and cartilage, or could be used for in vitro differentiation of stem cells using mechanical clues.











Similar content being viewed by others
References
Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani M-H, Ramakrishna S (2008) Electrospun poly (ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 29(34):4532–4539
Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS (2009) Control of stem cell fate by physical interactions with the extracellular matrix. Cell stem cell 5(1):17–26
Amirsadeghi A, Jafari A, Eggermont LJ, Hashemi S-S, Bencherif SA, Khorram M (2020) Vascularization strategies for skin tissue engineering. Biomater Sci 8(15):4073–4094. https://doi.org/10.1039/D0BM00266F
Chan B, Leong K (2008) Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 17(4):467–479
Chen F-M, Liu X (2016) Advancing biomaterials of human origin for tissue engineering. Progress Polym Sci 53:86–168
Mondschein RJ, Kanitkar A, Williams CB, Verbridge SS, Long TE (2017) Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 140:170–188
Eltom A, Zhong G, Muhammad A (2019) Scaffold techniques and designs in tissue engineering functions and purposes: a review. Adv Mater Sci Eng. https://doi.org/10.1155/2019/3429527
O’brien FJ (2011) Biomaterials and scaffolds for tissue engineering. Mater Today 14(3):88–95
Yang Y, Wang K, Gu X, Leong KW (2017) Biophysical regulation of cell behavior—cross talk between substrate stiffness and nanotopography. Engineering 3(1):36–54
Buxboim A, Rajagopal K, Andre’EX B, Discher DE (2010) How deeply cells feel: methods for thin gels. J Phys Condens Matter 22(19):194116
Kandow CE, Georges PC, Janmey PA, Beningo KA (2007) Polyacrylamide hydrogels for cell mechanics: steps toward optimization and alternative uses. Methods Cell Biol 83:29–46
Fang J, Li P, Lu X, Fang L, Lü X, Ren F (2019) A strong, tough, and osteoconductive hydroxyapatite mineralized polyacrylamide/dextran hydrogel for bone tissue regeneration. Acta biomaterialia 88:503–513
Tse JR, Engler AJ (2010) Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol 47(1):10–16
Xu J, Sun M, Tan Y, Wang H, Wang H, Li P, Xu Z, Xia Y, Li L, Li Y (2017) Effect of matrix stiffness on the proliferation and differentiation of umbilical cord mesenchymal stem cells. Differentiation 96:30–39
Lee J, Abdeen AA, Zhang D, Kilian KA (2013) Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 34(33):8140–8148
Lee JP, Kassianidou E, MacDonald JI, Francis MB, Kumar S (2016) N-terminal specific conjugation of extracellular matrix proteins to 2-pyridinecarboxaldehyde functionalized polyacrylamide hydrogels. Biomaterials 102:268–276
Pandamooz S, Jafari A, Salehi MS, Jurek B, Ahmadiani A, Safari A, Hassanajili S, Borhani-Haghighi A, Dianatpour M, Niknejad H (2019) Substrate stiffness affects the morphology and gene expression of epidermal neural crest stem cells in a short term culture. Biotech Bioeng 117(2):305–317
Gribova V, Crouzier T, Picart C (2011) A material’s point of view on recent developments of polymeric biomaterials: control of mechanical and biochemical properties. J Mater Chem 21(38):14354–14366
Li G, Zhao Y, Zhang L, Gao M, Kong Y, Yang Y (2016) Preparation of graphene oxide/polyacrylamide composite hydrogel and its effect on Schwann cells attachment and proliferation. Colloid Surf B Biointerfaces 143:547–556
Kundu B, Kundu SC (2012) Silk sericin/polyacrylamide in situ forming hydrogels for dermal reconstruction. Biomaterials 33(30):7456–7467
Ranganathan S, Balagangadharan K, Selvamurugan N (2019) Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int J Biol Macromol 133:354–364
Nafea EH, Poole-Warren LA, Martens PJ (2015) Bioactivity of permselective PVA hydrogels with mixed ECM analogues. J Biomed Mater Res Part A 103(12):3727–3735
Jafari A, Amirsadeghi A, Hassanajili S, Azarpira N (2020) Bioactive antibacterial bilayer PCL/gelatin nanofibrous scaffold promotes full-thickness wound healing. Int J Pharm 583:119413
Discher DE, Janmey P, Wang Y-l (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310(5751):1139–1143
Madl CM, Heilshorn SC, Blau HM (2018) Bioengineering strategies to accelerate stem cell therapeutics. Nature 557(7705):335
Langhans SA (2018) Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharm 9:6
Hamri S, Lerari D, Sehailia M, Dali-Youcef B, Bouchaour T, Bachari K (2018) Prediction of equilibrium swelling ratio on synthesized polyacrylamide hydrogel using central composite design modeling. Int J Plast Technol 22(2):247–261
Sabbagh F, Muhamad II, Nazari Z, Mobini P, Taraghdari SB (2018) From formulation of acrylamide-based hydrogels to their optimization for drug release using response surface methodology. Mater Sci Eng C 92:20–25
Mehrali M, Thakur A, Kadumudi FB, Pierchala MK, Cordova JAV, Shahbazi M-A, Mehrali M, Pennisi CP, Orive G, Gaharwar AK (2019) Pectin methacrylate (PEMA) and gelatin-based hydrogels for cell delivery: converting waste materials into biomaterials. ACS Appl Mater Interfaces 11(13):12283–12297
Qin Q, Tang Q, Li Q, He B, Chen H, Wang X, Yang P (2014) Incorporation of H3PO4 into three-dimensional polyacrylamide-graft-starch hydrogel frameworks for robust high-temperature proton exchange membrane fuel cells. Int J Hydrog Energy 39(9):4447–4458
Mandal BB, Kapoor S, Kundu SC (2009) Silk fibroin/polyacrylamide semi-interpenetrating network hydrogels for controlled drug release. Biomaterials 30(14):2826–2836
Zhang L, Liu J, Zheng X, Zhang A, Zhang X, Tang K (2019) Pullulan dialdehyde crosslinked gelatin hydrogels with high strength for biomedical applications. Carbohydrate Polym 216:45–53
Sobhanian P, Khorram M, Hashemi S-S, Mohammadi A (2019) Development of nanofibrous collagen-grafted poly (vinyl alcohol)/gelatin/alginate scaffolds as potential skin substitute. Int J Biol Macromol 130:977–987
Carvalho IC, Mansur HS (2017) Engineered 3D-scaffolds of photocrosslinked chitosan-gelatin hydrogel hybrids for chronic wound dressings and regeneration. Mater Sci Eng C 78:690–705
Samanta HS, Ray SK (2014) Synthesis, characterization, swelling and drug release behavior of semi-interpenetrating network hydrogels of sodium alginate and polyacrylamide. Carbohydr Polym 99:666–678
Cong HP, Wang P, Yu SH (2014) Highly elastic and superstretchable graphene oxide/polyacrylamide hydrogels. Small 10(3):448–453
Yan X, Yang J, Chen F, Zhu L, Tang Z, Qin G, Chen Q, Chen G (2018) Mechanical properties of gelatin/polyacrylamide/graphene oxide nanocomposite double-network hydrogels. Comp Sci Tech 163:81–88
Ghobashy MM, El-Damhougy BK, Nady N, Abd El-Wahab H, Naser A, Abdelhai F (2018) Radiation crosslinking of modifying super absorbent (polyacrylamide/gelatin) hydrogel as fertilizers carrier and soil conditioner. J Polym Environ 26(9):3981–3994
Loh QL, Choong C (2013) Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev 19(6):485–502
Park J, Kim D (2010) Effect of polymer solution concentration on the swelling and mechanical properties of glycol chitosan superporous hydrogels. J Appl Polym Sci 115(6):3434–3441
Jaiswal M, Dinda AK, Gupta A, Koul V (2010) Polycaprolactone diacrylate crosslinked biodegradable semi-interpenetrating networks of polyacrylamide and gelatin for controlled drug delivery. Biomed Mater 5(6):065014
Zhang Y, Chen H, Li Y, Fang A, Wu T, Shen C, Zhao Y, Zhang G (2020) A transparent sericin-polyacrylamide interpenetrating network hydrogel as visualized dressing material. Polym Test 87:106517
Poursamar SA, Lehner AN, Azami M, Ebrahimi-Barough S, Samadikuchaksaraei A, Antunes APM (2016) The effects of crosslinkers on physical, mechanical, and cytotoxic properties of gelatin sponge prepared via in-situ gas foaming method as a tissue engineering scaffold. Mater Sci Eng C 63:1–9
Huang J, Zhao L, Wang T, Sun W, Tong Z (2016) Nir-triggered rapid shape memory PAM–GO–gelatin hydrogels with high mechanical strength. ACS Appl Mater Interfaces 8(19):12384–12392
Hu X, Feng L, Xie A, Wei W, Wang S, Zhang J, Dong W (2014) Synthesis and characterization of a novel hydrogel: salecan/polyacrylamide semi-IPN hydrogel with a desirable pore structure. J Mater Chem B 2(23):3646–3658
Meng Z, Thakur T, Chitrakar C, Jaiswal MK, Gaharwar AK, Yakovlev VV (2017) Assessment of local heterogeneity in mechanical properties of nanostructured hydrogel networks. Acs Nano 11(8):7690–7696
Wang Q, Hou R, Cheng Y, Fu J (2012) Super-tough double-network hydrogels reinforced by covalently compositing with silica-nanoparticles. Soft Matter 8(22):6048–6056
Chanda M (2013) Introduction to polymer science and chemistry: a problem-solving approach. CRC Press, Florida
Maitra J, Shukla VK (2014) Cross-linking in hydrogels-a review. Am J Polym Sci 4(2):25–31
Ou K, Dong X, Qin C, Ji X, He J (2017) Properties and toughening mechanisms of PVA/PAM double-network hydrogels prepared by freeze-thawing and anneal-swelling. Mater Sci Eng C 77:1017–1026
Wang B, Xiao X, Zhang Y, Liao L (2019) High strength dual-crosslinked hydrogels with photo-switchable color changing behavior. Eur Polym J 116:545–553. https://doi.org/10.1016/j.eurpolymj.2019.04.035
Mohan YM, Murthy PK, Raju KM (2005) Synthesis, characterization and effect of reaction parameters on swelling properties of acrylamide–sodium methacrylate superabsorbent copolymers. React Funct Polym 63(1):11–26
Bajpai A, Giri A (2003) Water sorption behaviour of highly swelling (carboxy methylcellulose-g-polyacrylamide) hydrogels and release of potassium nitrate as agrochemical. Carbohydr Polym 53(3):271–279
Merlin DL, Sivasankar B (2009) Synthesis and characterization of semi-interpenetrating polymer networks using biocompatible polyurethane and acrylamide monomer. Eur Polym J 45(1):165–170
Li X, Wu W, Wang J, Duan Y (2006) The swelling behavior and network parameters of guar gum/poly (acrylic acid) semi-interpenetrating polymer network hydrogels. Carbohydr Polym 66(4):473–479
Singhal R, Tomar RS, Nagpal A (2009) Effect of cross-linker and initiator concentration on the swelling behaviour and network parameters of superabsorbent hydrogels based on acrylamide and acrylic acid. Int J Plast Technol 13(1):22
Zhou C, Wu Q, Zhang Q (2011) Dynamic rheology studies of in situ polymerization process of polyacrylamide–cellulose nanocrystal composite hydrogels. Colloid Polym Sci 289(3):247–255
Lahooti B, Khorram M, Karimi G, Mohammadi A, Emami A (2016) Modeling and optimization of antibacterial activity of the chitosan-based hydrogel films using central composite design. J Biomed Mater Res Part A 104(10):2544–2553
Sun G, Zhang X, Bao Z, Lang X, Zhou Z, Li Y, Feng C, Chen X (2018) Reinforcement of thermoplastic chitosan hydrogel using chitin whiskers optimized with response surface methodology. Carbohydr Polym 189:280–288
Zhao L, Li Q, Xu X, Kong W, Li X, Su Y, Yue Q, Gao B (2016) A novel Enteromorpha based hydrogel optimized with Box-Behnken response surface method: synthesis, characterization and swelling behaviors. Chem Eng J 287:537–544
Kumru B, Molinari V, Shalom M, Antonietti M, Schmidt BV (2018) Tough high modulus hydrogels derived from carbon-nitride via an ethylene glycol co-solvent route. Soft Matter 14(14):2655–2664
Liu X, Duan L, Gao G (2017) Rapidly self-recoverable and fatigue-resistant hydrogels toughened by chemical crosslinking and hydrophobic association. Eur Polym J 89:185–194
Li J, Liu H, Wang C, Huang G (2017) A facile method to fabricate hybrid hydrogels with mechanical toughness using a novel multifunctional cross-linker. RSC Adv 7(56):35311–35319
Caliari SR, Burdick JA (2016) A practical guide to hydrogels for cell culture. Nature Methods 13(5):405
Davidenko N, Schuster CF, Bax DV, Farndale RW, Hamaia S, Best SM, Cameron RE (2016) Evaluation of cell binding to collagen and gelatin: a study of the effect of 2D and 3D architecture and surface chemistry. J Mater Sci Mater Med 27(10):148
Hu X, Wang Y, Zhang L, Xu M (2017) Morphological and mechanical properties of tannic acid/PAAm semi-IPN hydrogels for cell adhesion. Polym Test 61:314–323
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jafari, A., Hassanajili, S., Ghaffari, F. et al. Modulating the physico-mechanical properties of polyacrylamide/gelatin hydrogels for tissue engineering application. Polym. Bull. 79, 1821–1842 (2022). https://doi.org/10.1007/s00289-021-03592-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03592-2