Abstract
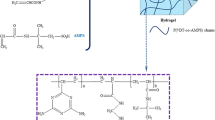
Recently, 3D cryogels with the interconnected macropore structure have gained great attention owing to their immense application potential in wastewater cleanup. Here, we fabricated a pure poly(vinyl imidazole) cryogel through improved cryogelation method, adding the pre-freezing–thawing step, which reduced the temperature difference between the different positions of the precursor solution during reaction. Compression experiments showed that the prepared 3D cryogel featured a robust underwater elastic performance. The adsorption capacity of Cu(II), Ni(II), Zn(II), Co(II), Pb(II), and Cd(II) ions from aqueous solutions by the poly(vinyl imidazole) cryogel was explored. The results showed the specific adsorption of Cu(II), in which the adsorption capacity and removal efficiency reached 148 mg/g and 99.99%, respectively. The adsorption capacity was 58 times higher than that of the reported poly(vinyl imidazole) cryogel which was prepared using hydroxyethyl methacrylate as the comonomer frameworks. In the reusability studies, the cryogel exhibited accumulating Cu(II) ions performance and excellent regeneration capability, making it a promising adsorbent for Cu(II) removal.







Similar content being viewed by others
References
Mittal A, Malviya A, Kaur D, Mittal J, Kurup L (2007) Studies on the adsorption kinetics and isotherms for the removal and recovery of Methyl Orange from wastewaters using waste materials. J Hazard Mater 148:229–240. https://doi.org/10.1016/j.jhazmat.2007.02.028
Huang H, He D, Tang Y, Guo Y, Li P, Qv W, Deng F, Lu F (2018) Adsorption of hexavalent chromium from an aqueous phase by hydroxypropyl methylcellulose modified with diethylenetriamine. J Chem Eng Data 64:98–106. https://doi.org/10.1021/acs.jced.8b00607
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Ray J, Jana S, Bhanja SK, Tripathy T (2018) Efficient removal of Co(II), Ni(II), and Zn(II) metal ions from binary and ternary solutions using a pH responsive bifunctional graft copolymer. Colloid Polym Sci 296:1275–1291. https://doi.org/10.1007/s00396-018-4345-4
Guclu G, Al E, Emik S, Iyim TB, Ozgümüş S, Ozyürek M (2009) Removal of Cu2+ and Pb2+ ions from aqueous solutions by starch-graft-acrylic acid/montmorillonite superabsorbent nanocomposite hydrogels. Polym Bull 65:333–346. https://doi.org/10.1007/s00289-009-0217-x
Guo Z, Li DD, Luo XK, Li YH, Zhao QN, Li MM, Zhao YT, Sun TS, Ma C (2017) Simultaneous determination of trace Cd(II), Pb(II) and Cu(II) by differential pulse anodic stripping voltammetry using a reduced graphene oxide-chitosan/poly-l-lysine nanocomposite modified glassy carbon electrode. J Colloid Interface Sci 490:11–22. https://doi.org/10.1016/j.jcis.2016.11.006
Ajji Z, Ali AM (2010) Separation of copper ions from iron ions using PVA-g-(acrylic acid/N-vinyl imidazole) membranes prepared by radiation-induced grafting. J Hazard Mater 173:71–74. https://doi.org/10.1016/j.jhazmat.2009.08.049
Tsai CY, Liu CW, Hsi HC, Lin KS, Lin YW, Lai LC, Weng TN (2019) Preparation of AgCl/TNTs nanocomposites for organic dyes and inorganic heavy metal removal. Environ Sci Pollut Res 26:22082–22096. https://doi.org/10.1007/s11356-019-05570-8
Dou X, Wang Q, Li Z, Ju J, Wang S, Hao L, Sui K, Xia Y, Tan Y (2019) Seaweed-derived electrospun nanofibrous membranes for ultrahigh protein adsorption. Adv Funct Mater 29:1905610. https://doi.org/10.1002/adfm.201905610
Fu Q, Si Y, Duan C, Yan Z, Liu L, Yu J, Ding B (2019) Highly carboxylated, cellular structured, and underwater superelastic nanofibrous aerogels for efficient protein separation. Adv Funct Mater 29:1808234. https://doi.org/10.1002/adfm.201808234
Memic A, Colombani T, Eggermont LJ, Rezaeeyazdi M, Steingold J, Rogers ZJ, Navare KJ, Mohammed HS, Bencherif SA (2019) Latest advances in cryogel technology for biomedical applications. Adv Thera 2:1800114. https://doi.org/10.1002/adtp.201800114
Jain A, Bajpai J, Bajpai AK, Mishra A (2019) Thermoresponsive cryogels of poly(2-hydroxyethyl methacrylate-co-N-isopropyl acrylamide) (P(HEMA-co-NIPAM)): fabrication, characterization and water sorption study. Polym Bull. https://doi.org/10.1007/s00289-019-02971-0
Gonul AA, Kose K, Kose DA (2018) Solvent effect on endosulfan adsorption onto polymeric arginine-methacrylate cryogels. Environ Sci Pollut Res 25:25458–25467. https://doi.org/10.1007/s11356-018-2531-z
Peng Y, Huang H, Zhang Y, Kang C, Chen S, Song L, Liu D, Zhong C (2018) A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat Commun 9:187. https://doi.org/10.1038/s41467-017-02600-2
Savina IN, Gun’ko VM, Turov VV, Dainiak M, Phillips GJ, Galaev IY, Mikhalovsky SV (2011) Porous structure and water state in cross-linked polymer and protein cryo-hydrogels. Soft Matter 7:4276–4283. https://doi.org/10.1039/c0sm01304h
Plieva FM, Karlsson M, Aguilar M-R, Gomez D, Mikhalovsky S, Galaev IY (2005) Pore structure in supermacroporous polyacrylamide based cryogels. Soft Matter 1:303–309. https://doi.org/10.1039/b510010k
Lin S, Sangaj N, Razafiarison T, Zhang C, Varghese S (2011) Influence of physical properties of biomaterials on cellular behavior. Pharm Res-dordr 28:1422–1430. https://doi.org/10.1007/s11095-011-0378-9
Kirsebom H, Topgaard D, Galaev IY, Mattiasson B (2010) Modulating the porosity of cryogels by influencing the nonfrozen liquid phase through the addition of inert solutes. Langmuir 26:16129–16133. https://doi.org/10.1021/la102917c
Savina IN, Ingavle GC, Cundy AB, Mikhalovsky SV (2016) A simple method for the production of large volume 3D macroporous hydrogels for advanced biotechnological, medical and environmental applications. Sci Rep-UK 6:21154. https://doi.org/10.1038/srep21154
Ingavle G, Baillie L, Davies N, Beaton N, Zheng Y, Mikhalovsky S, Sandeman S (2018) Bioinspired detoxification of blood: The efficient removal of anthrax toxin protective antigen using an extracorporeal macroporous adsorbent device. Sci Rep-UK 8:7518. https://doi.org/10.1038/s41598-018-25678-0
Andac M, Galaev IY, Denizli A (2013) Molecularly imprinted poly(hydroxyethyl methacrylate) based cryogel for albumin depletion from human serum. Colloid surface B 109:259–265. https://doi.org/10.1016/j.colsurfb.2013.03.054
Badhe RV, Bijukumar D, Chejara DR, Mabrouk M, Choonara YE, Kumar P, du Toit LC, Kondiah PPD, Pillay V (2017) A composite chitosan-gelatin bi-layered, biomimetic macroporous scaffold for blood vessel tissue engineering. Carbohyd Polym 157:1215–1225. https://doi.org/10.1016/j.carbpol.2016.09.095
Choudhury S, Connolly D, White B (2015) Supermacroporous polyHIPE and cryogel monolithic materials as stationary phases in separation science: a review. Anal Methods-UK 7:6967–6982. https://doi.org/10.1039/c5ay01193k
Yue Y, Mayes RT, Gill G, Kuo L-J, Wood J, Binder A, Brown S, Dai S (2015) Macroporous monoliths for trace metal extraction from seawater. RSC Adv 5:50005–50010. https://doi.org/10.1039/c5ra02131f
Cam T, Osman B, Kara A, Demirbel E, Besirli N, Irez G (2014) Potentiometric, kinetic, and thermodynamic investigations into Cu2+ ion binding properties of vinyl imidazole containing IMAC adsorbent. J Appl Polym Sci 131:39751. https://doi.org/10.1002/app.39751
Tekin K, Uzun L, Sahin CA, Bektas S, Denizli A (2011) Preparation and characterization of composite cryogels containing imidazole group and use in heavy metal removal. React Funct Polym 71:985–993. https://doi.org/10.1016/j.reactfunctpolym.2011.06.005
Firlak M, Cubuk S, Yetimoglu EK, Kahraman MV (2011) Uptake of Pb2+ using N-vinyl imidazole based uniform porous hydrogels. Sep Sci Technol 46:1984–1993. https://doi.org/10.1080/01496395.2011.579080
Al Lafi AG, Ajji Z (2017) Radiation grafting of acrylic acid and N-vinyl imidazole onto polyethylene films for lead-ion removal: a two-dimensional correlation infrared spectroscopy investigation. J Appl Polym Sci 134:44781. https://doi.org/10.1002/app.44781
Geng H (2018) A one-step approach to make cellulose-based hydrogels of various transparency and swelling degrees. Carbohydr Polym 186:208–216. https://doi.org/10.1016/j.carbpol.2018.01.031
Kim KH, Oh Y, Islam MF (2012) Graphene coating makes carbon nanotube aerogels superelastic and resistant to fatigue. Nat Nanotechnol 7:562–566. https://doi.org/10.1038/nnano.2012.118
Qiu L, Liu JZ, Chang SL, Wu Y, Li D (2012) Biomimetic superelastic graphene-based cellular monoliths. Nat Commun 3:1241. https://doi.org/10.1038/ncomms2251
Gao HL, Zhu YB, Mao LB, Wang FC, Luo XS, Liu YY, Lu Y, Pan Z, Ge J, Shen W, Zheng YR, Xu L, Wang LJ, Xu WH, Wu HA, Yu SH (2016) Super-elastic and fatigue resistant carbon material with lamellar multi-arch microstructure. Nat Commun 7:12920. https://doi.org/10.1038/ncomms12920
Arpa C, Bereli N, Ozdil E, Bektas S, Denizli A (2010) Reactive green HE-4BD functionalized supermacroporous poly(hydroxyethyl methacrylate) cryogel for heavy metal removal. J Appl Polym Sci 118:2208–2215. https://doi.org/10.1002/app.32592
Cimen D, Denizli A (2012) Immobilized metal affinity monolithic cryogels for cytochrome c purification. Colloids Surf B Biointerfaces 93:29–35. https://doi.org/10.1016/j.colsurfb.2011.11.058
Cimen D, Türkmen D, Denizli A (2016) Poly-(l)-histidine immobilized cryogels for lysozyme purification. Adsorpt Sci Technol 34:469–487. https://doi.org/10.1177/0263617416664453
Zhang L, Lu H, Yu J, Fan Y, Yang Y, Ma J, Wang Z (2018) Synthesis of lignocellulose-based composite hydrogel as a novel biosorbent for Cu2+ removal. Cellulose 25:7315–7328. https://doi.org/10.1007/s10570-018-2077-8
Giles CH, Smith D, Huitson A (1974) A general treatment and classification of the solute adsorption isotherm part. II. experimental interpretation. J Colloid Interf Sci 47:755–765. https://doi.org/10.1016/0021-9797(74)90253-7
Chen J, Zhang W, Li X (2015) Adsorption of Cu(II) ion from aqueous solutions on hydrogel prepared from Konjac glucomannan. Polym Bull 73:1965–1984. https://doi.org/10.1007/s00289-015-1588-9
Saleh TA (2015) Isotherm, kinetic, and thermodynamic studies on Hg(II) adsorption from aqueous solution by silica- multiwall carbon nanotubes. Environ Sci Pollut Res 22:16721–16731. https://doi.org/10.1007/s11356-015-4866-z
Liao J, Huang H (2019) Magnetic chitin hydrogels prepared from Hericium erinaceus residues with tunable characteristics: a novel biosorbent for Cu2+ removal. Carbohydr Polym 220:191–201. https://doi.org/10.1016/j.carbpol.2019.05.074
Tang J, Song Y, Zhao F, Spinney S, da Silva BJ, Tam KC (2019) Compressible cellulose nanofibril (CNF) based aerogels produced via a bio-inspired strategy for heavy metal ion and dye removal. Carbohydr Polym 208:404–412. https://doi.org/10.1016/j.carbpol.2018.12.079
Mohammed N, Grishkewich N, Berry RM, Tam KC (2015) Cellulose nanocrystal-alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 22:3725–3738. https://doi.org/10.1007/s10570-015-0747-3
Tang J, Sisler J, Grishkewich N, Tam KC (2017) Functionalization of cellulose nanocrystals for advanced applications. J Colloid Interf Sci 494:397–409. https://doi.org/10.1016/j.jcis.2017.01.077
Kolodynska D (2011) Green complexing agent-EDDS in removal of heavy metal ions on strongly basic anion exchangers. Desalination 280:44–57. https://doi.org/10.1016/j.desal.2011.06.060
Vincent T, Parodi A, Guibal E (2008) Immobilization of Cyphos IL-101 in biopolymer capsules for the synthesis of Pd sorbents. React Funct Polym 68:1159–1169. https://doi.org/10.1016/j.reactfunctpolym.2008.04.001
Qu W, He D, Guo Y, Tang Y, Song RJ (2019) Characterization of modified Alternanthera philoxeroides by diethylenetriamine and its application in the adsorption of copper(II) ions in aqueous solution. Environ Sci Pollut Res 26:21189–21200. https://doi.org/10.1007/s11356-019-05472-9
O’Connell DW, Birkinshaw C, O’Dwyer TF (2006) A chelating cellulose adsorbent for the removal of Cu(II) from aqueous solutions. J Appl Polym Sci 99:2888–2897. https://doi.org/10.1002/app.22568
Sekhavat Pour Z, Ghaemy M (2015) Removal of dyes and heavy metal ions from water by magnetic hydrogel beads based on poly(vinyl alcohol)/carboxymethyl starch-g-poly(vinyl imidazole). RSC Adv 5:64106–64118. https://doi.org/10.1039/c5ra08025h
Ren Y, Zhang J, Guo J, Chen F, Yan F (2017) Porous poly(Ionic Liquid) membranes as efficient and recyclable absorbents for heavy metal ions. Macromol Rapid Commun 38(14):1700151. https://doi.org/10.1002/marc.201700151
Chen Y, Zhao W, Yang X, Li Y (2018) Efficient removal of heavy metal ions from aqueous solution by a novel poly (1-vinylimidazole) chelate resin. Polym Bull 76:1081–1097. https://doi.org/10.1007/s00289-018-2426-7
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Grant Nos. 21965039 and 21464017), Natural Science Foundation of Yunnan Province (Grant Nos. 2014FB139 and 2018FD017), and Science Research Foundation of Yunnan Education Bureau (Grant No. 2014Z046).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhong, T., Feng, X., Sun, L. et al. Highly effective adsorption of copper ions by poly(vinyl imidazole) cryogels. Polym. Bull. 78, 5873–5890 (2021). https://doi.org/10.1007/s00289-020-03413-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03413-y