Abstract
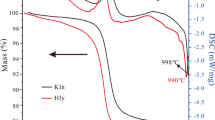
Kaolinite particles were exfoliated by the intercalation and subsequent deintercalation of urea in the interlayer of kaolinite. The exfoliated kaolinite particles were used to prepare a novel poly(ethylene oxide)/kaolinite (PEO/kaolinite) composite. The resulting exfoliated kaolinite and PEO/kaolinite composites were characterized by X-ray diffraction (XRD), Fourier transfer infrared spectroscopy (FTIR), scanning electron microscopy (SEM), differential scanning calorimetry (DSC), ion conductivity test, thermogravimetry (TG) analysis and mechanical test. XRD results showed that the 001 diffraction of kaolinite in the XRD pattern almost disappeared after exfoliation treatment. FTIR spectra showed that the inner surface hydroxyls of kaolinite decreased after exfoliating kaolinite layers. SEM micrographs confirmed the exfoliation of kaolinite particles and showed a good dispersion of exfoliated kaolinite in PEO matrix. A gradual decrease in PEO crystallinity in PEO/kaolinite composites was observed with the increment of the exfoliated kaolinite concentration by DSC test. The ion conductivity of the composites gradually increased with exfoliated kaolinite content and reached 6.1 × 10−5 (S/cm) at a filler concentration of 20 wt%. The formation of amorphous region around the exfoliated kaolinite was beneficial for the Li+ ion conduction. Thermogravimetry analysis showed that the decomposition temperature of PEO matrix at weight loss of 10 and 50 wt% was increased when exfoliated kaolinite was introduced into PEO matrix. In addition, the mechanical properties of PEO matrix were also enhanced when exfoliated kaolinite content in PEO matrix was no more than 15 wt%.











Similar content being viewed by others
References
Bhattacharyya AJ, Fleig J, Guo YG, Maier J (2005) Local conductivity effects in polymer electrolytes. Adv Mater 17:2630–2634. doi:10.1002/adma.200500926
Marzantowicz M, Dygas JR, Krok F, Lasinska A, Florjanczyk Z, Zygadlo-Monikowska E, Affek A (2005) Crystallization and melting of PEO:LiTFSI polymer electrolytes investigated simultaneously by impedance spectroscopy and polarizing microscopy. Electrochim Acta 50:3969–3977. doi:10.1016/j.electacta.2005.02.053
Marzantowicz M, Dygas JR, Krok F, Nowinski JL, Tomaszewska A, Florjanczyk Z, Zygadlo-Monikowska E (2006) Crystalline phases, morphology and conductivity of PEO:LiTFSI electrolytes in the eutectic region. J Power Sources 159:420–430. doi:10.1016/j.jpowsour.2006.02.044
Munshi MZA, Owens BB (1988) Performance of polymer electrolyte cells at +25 to +100 °C. Solid State Ionics 26:41–46. doi:10.1016/0167-2738(88)90244-5
Huq R, Farrington GC, Koksbang R, Tonder PE (1992) Influence of plasticizers on the electrochemical and chemical stability of a Li+ polymer electrolyte. Solid State Ionics 57:277–283. doi:10.1016/0167-2738(92)90159-M
Loyens W, Maurer FHJ, Jannasch P (2005) Melt-compounded salt-containing poly(ethylene oxide)/clay nanocomposites for polymer electrolyte membranes. Polymer 46:7334–7345. doi:10.1016/j.polymer.2005.06.006
Abraham TN, Siengchin S, Ratna D, Karger-Kocsis J (2010) Effect of modified layered silicates on the confined crystalline morphology and thermomechanical properties of poly(ethylene oxide) nanocomposites. J Appl Polym Sci 118:1297–1305. doi:10.1002/app
Zhang H, Maitra P, Wunder SL (2008) Preparation and characterization of composite electrolytes based on PEO(375)-grafted fumed silica. Solid State Ionics 178:1975–1983. doi:10.1016/j.ssi.2007.11.021
Xie J, Duan RG, Han Y, Kerr JB (2004) Morphological, rheological and electrochemical studies of poly(ethylene oxide) electrolytes containing fumed silica nanoparticles. Solid State Ionics 175:755–758. doi:10.1016/j.ssi.2003.10.021
Ruiz-Hitzky E, Aranda P (1990) Polymer-salt intercalation complexes in layer silicates. Adv Mater 2:545–547. doi:10.1002/adma.19900021108
Kim S, Hwang EJ, Jung Y, Hana M, Park SJ (2008) Ionic conductivity of polymeric nanocomposite electrolytes based on poly(ethylene oxide) and organo-clay materials. Colloid Surf A 313–314:216–219. doi:10.1016/j.colsurfa.2007.04.097
Zhang YG, Zhao Y, Gosselink D, Chen P (2015) Synthesis of poly(ethylene-oxide)/nanoclay solid polymer electrolyte for all solid-state lithium/sulfur battery. (report). Ionics 21:381–385. doi:10.1007/s11581-014-1176-2
Chen W, Xu Q, Yuan RZ (2000) Effect on ionic conductivity with modification of polymethylmethacrylate in poly(ethylene oxide)-layered silicate nanocomposites (PLSN). Mat Sci Eng B-Solid 77:15–18
Itagaki T, Matsumura A, Kato M, Usuki A, Kuroda KJ (2001) Preparation of kaolinite–nylon6 composites by blending nylon6 and a kaolinite–nylon6 intercalation compound. Mater Sci Lett 20:1483–1484. doi:10.1023/A:1017918228163
Zhang X, Xu Z (2007) The effect of microwave on preparation of kaolinite/dimethylsulfoxide composite during intercalation process. Mater Lett 61:1478–1482. doi:10.1016/j.matlet.2006.07.057
Stellano M, Turturro A, Riani P, Montanari T, Finocchio E, Ramis G, Busca G (2010) Bulk and surface properties of commercial kaolins. Appl Clay Sci 48:446–454. doi:10.1016/j.clay.2010.02.002
Wang YL, Lee BS, Chang KC, Chiu HC, Lin FH, Lin CP (2007) Characterization, fluoride release and recharge properties of polymer-kaolinite nanocomposite. Compos Sci Technol 67:3409–3416. doi:10.1016/j.compscitech.2007.03.018
Bahramian AR, Kokabi M, Famili MHN, Beheshty MH (2008) High temperature ablation of kaolinite layered silicate/phenolic resin/asbestos cloth nanocomposite. J Hazard Mater 150:136–145. doi:10.1016/j.jhazmat.2007.04.104
Villanueva MP, Cabedo L, Lagaron JM, Gimenez EJ (2010) Comparative study of nanocomposites of polyolefin compatibilizers containing kaolinite and montmorillonite organoclays. Appl Polym Sci 115:1325–1335. doi:10.1002/app.30278
Mpofu P, Addai-Mensah J, Ralston J (2003) Investigation of the effect of polymer structure type on flocculation, rheology and dewatering behaviour of kaolinite dispersions. Int J Miner Process 71:247–268. doi:10.1016/S0301-7516(03)00062-0
Mpofu P, Addai-Mensah J, Ralston J (2004) Temperature influence of nonionic polyethylene oxide and anionic polyacrylamide on flocculation and dewatering behavior of kaolinite dispersions. J Colloid Interface Sci 271:145–156. doi:10.1016/j.jcis.2003.09.042
Liang L, Tan JK, Peng YL, Xia WC, Xie GY (2016) The role of polyaluminum chloride in kaolinite aggregation in the sequent coagulation and flocculation process. J Colloid Interf Sci 468:57–61
Gardolinski JE, Carrera LCM (2000) Layered polymer-kaolinite nanocomposites. J Mater Sci 35:3113–3119
Valáškováa M, Riedera M, Matějkaa V, Čapkováa P, Slívab A (2007) Exfoliation/delamination of kaolinite by low-temperature washing of kaolinite-urea intercalates. Appl Clay Sci 35:108–118. doi:10.1016/j.clay.2006.07.001
Liu YL, Wei WL, Hsu KY, Ho WH (2004) Thermal stability of epoxy-silica hybrid materials by thermogravimetric analysis. Thermochim Acta 412:139–147. doi:10.1016/j.tca.2003.09.004
Yuan P, Tan DY, Annabi-Bergaya F, Yan WC, Liu D, Liu ZW (2013) From platy kaolinite to aluminosilicate nanoroll via one-step delamination of kaolinite: effect of the temperature of intercalation. Appl Clay Sci 83–84:68–76
Xue B, Yang K, Wang XY, Chi QW, Jiang YS (2016) The role of potassium chlorate on expansion of dickite layers and the preparation of a novel TiO2 impregnated dickite photocatalyst using expanded dickite as carrier. Rsc Adv 6:9803–9811. doi:10.1039/c5ra23615k
Zhang S, Liu QF, Cheng HF, Zeng FG (2015) Combined experimental and theoretical investigation of interactionsbetween kaolinite inner surface and intercalated dimethyl sulfoxide. Appl Surf Sci 331:234–240
Ratna D, Divekar S, Samui AB, Chakraborty BC, Banthia AK (2006) Poly(ethylene oxide)/clay nanocomposite: thermomechanical properties and morphology. Polymer 47:4068–4074. doi:10.1016/j.polymer.2006.02.040
Reddy MJ, Chu PP, Rao UVS (2006) Study of multiple interactions in mesoporous composite PEO electrolytes. J Power Sources 158:614–619. doi:10.1016/j.jpowsour.2005.10.008
Burgaz E, Yazici M, Kapusuz M, Alisir SH, Ozcan H (2014) Prediction of thermal stability, crystallinity and thermomechanical properties of poly(ethylene oxide)/clay nanocomposites with artificial neural networks. Thermochim Acta 575:159–166
Aranda P, Ruiz-Hitzky E (1992) Poly(ethylene oxide)-silicate intercalation materials. Chem Mater 4:1395–1403. doi:10.1021/cm00024a048
Papke BL, Ratner MA, Shriver DF (1981) Vibrational spectroscopy and structure of polymer electrolytes, poly(ethylene oxide) complexes of alkali metal salts. J Phys Chem Solids 42:493–500. doi:10.1016/0022-3697(81)90030-5
Aranguren MI, Mora E, DeGroot JV, Macosko CW (1992) Effect of reinforcing fillers on the rheology of polymer melts. J Rheol 36:1165–1182. doi:10.1122/1.550306
Wunderlich B (1980) Macromolecular physics. Academic Press, New York
Chen H-W, Chang F-C (2011) The novel polymer electrolyte nanocomposite composed of poly(ethylene oxide), lithium triflate and mineral clay. Polymer 42:9763–9769
Groenewoud WM (2001) Characterization of polymers by thermal analysis, Eerste Hervendreef 32, 5232 JK’s Hertogenbosch. Elsevier, The Netherlands, p 13
Bandara T, Mellander B-E, Albinsson I, Dissanayake M, Pitawala H (2009) Thermal and dielectric properties of PEO/EC/Pr4N + I—polymer electrolytes for possible applications in photo-electro chemical solar cells. J Solid State Electr 13:1227–1232. doi:10.1007/s10008-008-0655-7
Kiran Kumar K, Ravi M, Pavani Y, Bhavani S, Sharma AK, Narasimha Rao VVR (2014) Investigations on PEO/PVP/NaBr complexed polymer blend electrolytes for electrochemical cell applications. J Membrane Sci 454:200–211. doi:10.1016/j.memsci.2013.12.022
Huang YP, Lee MJ, Yang MK, Chen CW (2010) Montmorillonite particle alignment and crystallization and ion-conducting behavior of montmorillonite/poly(ethylene oxide) nanocomposites. Appl Clay Sci 49:163–169. doi:10.1016/j.clay.2010.04.021
Kim S, Hwang E-J, Park S-J (2008) An experimental study on the effect of mesoporous silica addition on ion conductivity of poly(ethylene oxide) electrolytes. Curr Appl Phys 8:729–731. doi:10.1016/j.cap.2007.04.028
Patra R, Suin S, Mandal D, Khatua BB (2015) Reduction of percolation threshold of multiwall carbon nanotube (MWCNT) in polystyrene (ps)/low-density polyethylene (LDPE)/mwcnt nanocomposites: an eco-friendly approach. Polym Composite 36:1574–1583. doi:10.1002/pc.23065
He LX, Tjong SC (2013) Low percolation threshold of graphene/polymer composites prepared by solvothermal reduction of graphene oxide in the polymer solution. Nanoscale Res Lett 8:1–7. doi:10.1186/1556-276X-8-132
McNeill IC, Zulfiqar M, Kousar T (1990) A detailed investigation of the products of the thermal degradation of polystyrene. Polym Degrad Stabil 28:131–151. doi:10.1016/0141-3910(90)90002-O
Zhang ZJ, Zhang LN, Li Y, Xu HD (2005) PNew fabricate of styrene-butadiene rubber/montmorillonite nanocomposites by anionic polymerization. Polymer 46:129–136. doi:10.1016/j.polymer.2004.11.008
Ye YP, Chen HB, Wu JS, Ye L (2007) High impact strength epoxy nanocomposites with natural nanotubes. Polymer 48:6426–6433. doi:10.1016/j.polymer.2007.08.035
Acknowledgements
Financial support from the Natural Scientific Foundation of China (Grant Nos. 41472035 and 51304080), the Project of Science and Technology Department (Jilin Province, Grant No. 20140204008SF), China Scholarship Council (CSC) and Undergraduate Innovative Experiment Project (2016B43288).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chi, Q., Zhen, R., Wang, X. et al. The role of exfoliated kaolinite on crystallinity, ion conductivity, thermal and mechanical properties of poly(ethylene oxide)/kaolinite composites. Polym. Bull. 74, 3089–3108 (2017). https://doi.org/10.1007/s00289-016-1884-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1884-z