Abstract
A characteristic of malaria in all its forms is the potential for superinfection (that is, multiple concurrent blood-stage infections). An additional characteristic of Plasmodium vivax malaria is a reservoir of latent parasites (hypnozoites) within the host liver, which activate to cause (blood-stage) relapses. Here, we present a model of hypnozoite accrual and superinfection for P. vivax. To couple host and vector dynamics for a homogeneously-mixing population, we construct a density-dependent Markov population process with countably many types, for which disease extinction is shown to occur almost surely. We also establish a functional law of large numbers, taking the form of an infinite-dimensional system of ordinary differential equations that can also be recovered by coupling expected host and vector dynamics (i.e. a hybrid approximation) or through a standard compartment modelling approach. Recognising that the subset of these equations that model the infection status of the human hosts has precisely the same form as the Kolmogorov forward equations for a Markovian network of infinite server queues with an inhomogeneous batch arrival process, we use physical insight into the evolution of the latter process to write down a time-dependent multivariate generating function for the solution. We use this characterisation to collapse the infinite-compartment model into a single integrodifferential equation (IDE) governing the intensity of mosquito-to-human transmission. Through a steady state analysis, we recover a threshold phenomenon for this IDE in terms of a parameter \(R_0\) expressible in terms of the primitives of the model, with the disease-free equilibrium shown to be uniformly asymptotically stable if \(R_0<1\) and an endemic equilibrium solution emerging if \(R_0>1\). Our work provides a theoretical basis to explore the epidemiology of P. vivax, and introduces a strategy for constructing tractable population-level models of malarial superinfection that can be generalised to allow for greater biological realism in a number of directions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Malaria is a parasitic, vector-borne disease with a staggering pulic health burden. The overwhelming majority of malaria cases (98%) are attributed to the parasite Plasmodium falciparum, particularly in Africa (WHO 2021). Plasmodium vivax, however, exhibits a broader geographical distribution, driving much of the malaria burden in South East Asia, the Americas, the Western Pacific and the Eastern Mediterranean (WHO 2021). While an estimated 4.5 million malaria cases were attributed to P. vivax in 2020 alone (WHO 2021), morbidity arising from P. vivax infections remains “obscure and insidious” (Battle and Kevin Baird 2021). The transmission of malaria parasites to humans is mediated by Anopheles mosquito vectors. During the course of a bloodmeal, an infected mosquito can transmit parasites (sporozoites) to a human host. Following a period of liver-stage development (exoerythrocytic schizogony), parasites are released into the bloodstream, giving rise to a blood-stage infection that is sustained by the replication of parasites in invaded red blood cells (Venugopal et al. 2020). A key epidemiological characteristic of malaria is the phenomenon of superinfection (that is, multiple concurrent blood-stage infections). Since the circulation and replication of (pre-existing) parasites in the bloodstream does not preclude further blood-stage infection, an individual can concurrently harbour multiple co-circulating broods of blood-stage parasites. In the context of P. falciparum, we define each blood-stage ‘brood’ to derive from a single infective bite. The interpretation of a blood-stage ‘brood’ for P. vivax is more nuanced, in light of its ability to cause relapsing infections following the accrual of a “hypnozoite reservoir” (White et al. 2014; White and Imwong 2012). Notably, a P. vivax parasite (sporozoite) injected into a human host has two possible fates: it either gives rise to a primary (blood-stage) infection within approximately 9 days of the bite itself (Mikolajczak 2015), or develops into a hypnozoite (Mueller et al. 2009). Hypnozoites undergo indeterminate latency periods, often lasting weeks or months, during which they are undetectable using standard techniques (Schäfer et al. 2021). The activation of each hypnozoite, however, can trigger an additional blood-stage infection, known as a relapse (Mueller et al. 2009). For P. vivax, each primary infection and relapse is defined to comprise a separate blood-stage brood.
We define the multiplicity of broods (MOB) to be the number of co-circulating blood-stage broods in a host at a given point in time, with superinfection taken to be a collective term for blood-stage infections with MOB \(>1\). As such, superinfection arises from temporally proximate reinfection (that is, infective bites) and, in the case of P. vivax, hypnozoite activation events. With epidemiological data indicating the preponderance of relapses over primary infections (Commons et al. 2020) and evidence of P. vivax superinfection even in the absence of reinfection (Popovici 2018), analysis of the statistics of superinfection for P. vivax warrants careful consideration of the hypnozoite reservoir.
The classical framework of malarial superinfection for P. falciparum, proposed initially by Macdonald (1950) and formulated mathematically by Bailey (1957), assumes independent clearance of each brood, without imposing an upper bound on the MOB. Under this setting, a natural construction to describe the within-host dynamics of superinfection is an infinite-server queue with a time dependent arrival rate given by the intensity of mosquito-to-human transmission (Dietz et al. 1974; Nedelman 1984; Smith and Hay 2009; Henry 2020). Hereafter, we refer to the mosquito-to-human transmission intensity, as quantified by the infective bite rate per human, as the force of reinfection (FORI). For P. vivax, introducing the additional assumption that the dynamics of each hypnozoite are governed by independent stochastic processes (White et al. 2014), we have recently extended this idea to characterise within-host and superinfection dynamics using an open network of infinite-server queues with geometrically-distributed batch arrivals at a time-dependent rate given by the FORI (Mehra et al. 2021, 2022). In Mehra et al. (2022), we derive a time-dependent generating function for the state of the queueing network, which can be inverted analytically to recover marginal distributions for MOB and the hypnozoite burden, amongst other quantities of epidemiological interest, on a within-host scale.
Mathematical modelling of superinfection and hypnozoite dynamics at the population-level can be challenging, owing to the potential difficulty of carrying out computational procedures for infinite-compartment models stratified by MOB and the hypnozoite burden (White 2018). As we note in Mehra et al. (2022), a common approach in the construction of transmission models of P. vivax has been to consolidate hypnozoite carriage into a single state, with an accompanying parametric form for the time to first relapse for hypnozoite-positive individuals (Aguas et al. 2012; Chamchod and Beier 2013; Ishikawa et al. 2003; Robinson 2015; Roy et al. 2013; White et al. 2016). The binarisation of hypnozoite carriage, however, obscures the relationship between transmission intensity and hypnozoite accrual: there is no variation in the risk of relapse based on hypnozoite density, the limitations of which are discussed in Mehra et al. (2022). Explicit variation in the hypnozoite burden is captured in the deterministic models of White et al. (2014), White (2018), and Anwar et al. (2022). While the ‘batch’ model of White (2018) captures ‘broods’ of hypnozoites in the liver, it ignores variation in parasite inoculum sizes. On the other hand, both Anwar et al. (2022) and White et al. (2014) take hypnozoite densities into account. To account for superinfection, White et al. (2014) employ a “pseudoequilibrium approximation” for the (blood-stage) infection recovery rate, adopting the functional form derived by Dietz et al. (1974). This functional form, which was derived in the absence of hypnozoite accrual, has been embedded in multiple transmission models for P. falciparum as a proxy for superinfection (Alonso et al. 2019; Gemperli et al. 2006; Reto Hagmann et al. 2003; Smith et al. 2007). However, we argue that this functional form, as embedded by White et al. (2014) in a model for P. vivax, is not appropriate in a model that takes hypnozoite accrual into account (see Appendix C for details). The multiscale model of Anwar et al. (2022) is intended to serve as a re-formulation of the infinite-compartment model proposed in White et al. (2014). To incorporate hypnozoite accrual in a simple population-level framework, it draws on the relapse rate, conditional on the absence of blood-stage infection, derived in Mehra et al. (2022), to derive a numerically tractable system of integrodifferential equations (IDEs). However, while the above-mentioned conditional relapse rate is derived under a framework that explicitly allows for superinfection, the population-level model of Anwar et al. (2022) does not have separate compartments for different values of the MOB (see Appendix D for details, and a proposed correction to Anwar et al. (2022) that has been adopted in subsequent work (Anwar et al. 2023)).
To the best of our knowledge, the thesis of Mehra (2022)—which forms of the basis of this paper—derives the first model that characterises both superinfection and hypnozoite dynamics for P. vivax. The model assumptions, building on the work of White et al. (2014), are detailed in Sect. 2. Here, we re-visit the queueing framework introduced in Mehra et al. (2022) to characterise within-host superinfection and hypnozoite dynamics as a function of the FORI; Mehra et al. (2022) is the source of the within-host model which we embed in the population. We then construct a density-dependent Markov population process to couple host and vector dynamics in a homogeneously-mixing population, whilst allowing for superinfection and the accrual of the hypnozoite reservoir in the absence of human demographics in Sect. 3, proving that disease extinction occurs almost surely (Theorem 3.1). Using the work of Barbour and Luczak (2012), we obtain a functional law of large numbers (FLLN), that takes the form of an infinite-dimensional system of ordinary differential equations (ODEs) (Sect. 3.2) for which the model of Bailey (1957) arises as a special case (Appendix E). By drawing on our previous analysis of the within-host model, we show that the FLLN can be reduced to a single IDE governing the time evolution of the FORI; the dynamics of superinfection and the hypnozoite reservoir in the human population can be recovered as a function of the FORI solving this IDE (Sect. 4.2). We then establish a threshold phenomenon for the reduced IDE, with the disease-free equilibrium shown to be uniformly asymptotically stable if \(R_0<1\), and an endemic equilibrium solution emerging iff \(R_0>1\) (Theorem 4.1, Sect. 4.3).
A recurrent theme in our analysis of population-level models is the utility of a physical understanding of the within-host model, governing the hypnozoite/MOB burden within a single human as a function of the intensity of mosquito-to-human transmission. In Sect. 5, we discuss how the ideas presented in this manuscript constitute a general strategy that can be employed to construct tractable population-level models of malarial superinfection, with appropriate constraints on the underlying model structure. In brief, the starting point of this approach is a within-host model characterised as a Markovian network of infinite server queues. Embedding this within-host model in a population framework—either as the FLLN limit for a Markov population process (Barbour and Luczak 2012); under a standard compartment modelling approach; or under a “hybrid approximation” (Henry 2020; Naasell 2013)—yields an infinite dimensional system of ODEs. Next, we make the observation that the host part of the population model has an identical form to the Kolmogorov forward differential equations for the queueing network. By exploiting the independence property of infinite server queues, we use physical insight to derive an integral expression for the infectivity of hosts to the vector population, conditional on the FORI. Substituting this integral expression back into the vector part of the population model then yields an integrodifferential framework that is amenable to further analysis.
2 Modelling within-host hypnozoite and superinfection dynamics using an open network of infinite server queues with batch arrivals
White et al. (2014) construct a within-host model for short-latency (tropical) strains of P. vivax, predicated on the following set of assumptions:
-
Each infective mosquito bite immediately gives rise to a primary (blood-stage) infection and establishes a batch of hypnozoites in the liver.
-
Hypnozoite batch sizes \(N_i\) (for the \(i^\text {th}\) bite) which are independent and identically-distributed (i.i.d.) across bites, are geometrically-distributed with probability mass function
$$\begin{aligned} P( N_i = n ) = \frac{1}{1 + \nu } \Big ( \frac{\nu }{1 + \nu } \Big )^n \end{aligned}$$(1)for \(n \in \mathbbm {Z}_{\ge 0}\) and mean \({{\,\mathrm{\mathbbm {E}}\,}}[N_i]=\nu \).
-
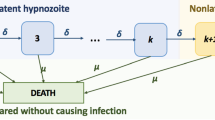
Each hypnozoite in the liver undergoes activation at constant rate \(\alpha \), which gives rise to the host suffering a (blood-stage) relapse; but each hypnozoite is also subject to death at constant rate \(\mu \).
-
Hypnozoites behave independently; that is, the dynamics of each of the hypnozoites is described by an independent stochastic process.
We extend the framework of White et al. (2014) to explicitly allow for superinfection. Specifically, we make the assumption that each blood-stage infection (relapse or primary) is naturally cleared at constant rate \(\gamma \), with independent dynamics for each hypnozoite and blood-stage infection; as such, the existence of a previous blood-stage infection does not preclude further blood- and liver-stage infections, nor alter the rate of clearance for subsequent blood-stage infections.
We first examine the within-host dynamics of superinfection and the hypnozoite reservoir as a function of the FORI. In Mehra et al. (2022), we construct an open network of infinite server queues with batch arrivals to concurrently describe hypnozoite accrual and the burden of blood-stage infection (allowing for superinfection) as a function of mosquito-to-human transmission intensity. The formulation of Mehra et al. (2022) can be extended to account for long-latency phenotypes, which are predominantly found in temperate regions (Mehra et al. 2020; White et al. 2014), and the administration of drug treatment at a pre-determined sequence of times. Here, we consider the simplest case of the model presented in Mehra et al. (2022), restricting our attention to short-latency phenotypes (characteristic of tropical transmission settings) (White et al. 2014) in the absence of drug treatment.
We begin by delineating the set of possible states that a single hypnozoite can occupy:
-
H indicates a hypnozoite that is currently present in the liver;
-
A indicates a hypnozoite that has activated to give rise to a relapse that is currently in progress;
-
C indicates a hypnozoite that has previously given rise to a relapse, which has since been cleared;
-
D indicates a hypnozoite that has died, rather than activating.
Hypnozoites in the liver (state H) undergo activation at rate \(\alpha \) and death at rate \(\mu \). Relapses (state A) are cleared from the bloodstream at rate \(\gamma \). This model, introduced in Mehra et al. (2022), is a simple extension of the short-latency model of White et al. (2014) that accounts for the clearance of blood-stage infection. A continuous-time Markov chain model that captures the above dynamics has non-zero transition rates
with absorbing states C and D.
We denote by \(p_s(t)\) the probability that a hypnozoite is in state \(s \in \{ H, A, C, D \}:= S_h\) at time t after inoculation. In practice, we would expect the mean duration of hypnozoite carriage \(1/(\alpha +\mu )\) to exceed the mean duration of each blood-stage infection \(1/\gamma \). It is straightforward to establish that when \(\alpha + \mu \ne \gamma \),
as in Eqs. (13) to (16) of Mehra et al. (2022).
Likewise, we delineate the state space for each primary infection:
-
P indicates a primary infection that is currently in progress;
-
PC indicates a primary infection that has been cleared from the bloodstream.
We assume that each bite necessarily triggers a primary infection that is cleared naturally from the bloodstream at the constant rate \(\gamma \), yielding a continuous-time Markov chain model with transition rates
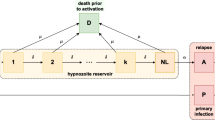
To embed our model for a single hypnozoite in an epidemiological framework, we construct an open network of infinite server queues, labelled H, A, C, D, P, PC (Fig. 1). The arrival process, comprising mosquito bites, is governed by non-homogeneous Poisson process with a time-dependent rate \(\lambda (t)\), such that
Each bite leads to the arrival of a single ‘individual’ in queue P (that is, a primary infection), in addition to a geometrically-distributed batch (with PMF (1)) in queue H (representing the hypnozoite reservoir). Hypnozoites in queue H follow the dynamics described above in moving to queues A, C or D. A primary infection in queue P moves to queue PC at rate \(\gamma \).
Denote by \(N_s(t)\) the number of ‘individuals’ (either hypnozoites or infections) in queue \(s \in \{ H, A, C, D, P, PC \}:= S\) at time t. From Eq. (39) of Mehra et al. (2022), given
the joint PGF for \(\textbf{N}(t) = (N_H(t), N_A(t), N_D(t), N_C(t), N_P(t), N_{PC}(t))\)
is guaranteed to converge in the domain \({\textbf{z}} \in [0, 1]^6\).
Schematic of the open network of infinite server queues governing the within-host hypnozoite and MOB burden, as a function of the intensity of mosquito-to-human transmission. Adapted from Figure 3 of Mehra et al. (2022) as a special case (short-latency hypnozoites, no drug treatment)
Explicit formulae for quantities of epidemiological interest—including marginal distributions for MOB and the hypnozoite burden; the proportion of recurrences that are expected to be relapses and the cumulative burden of blood-stage infection over time—are provided in Mehra et al. (2022). There the marginal distributions for MOB and the hypnozoite burden are expressed in terms of partial exponential Bell polynomials. We can equivalently formulate recurrence relations to compute marginal distributions of interest following the approach of Willmot and Drekic (2001), or compute joint distributions using the recurrence relations we derive in Mehra and Taylor (2023).
3 A Markov population process with countably many types
To couple vector and human dynamics, whilst accounting for superinfection and the hypnozoite reservoir in the absence of human demographics (that is, without allowing for births/deaths in the human population), we construct a density-dependent Markov population process with countably many types. We consider a closed, homogeneously mixing population, with fixed numbers of humans \(P_H\) and mosquitoes \(P_M\), where:
-
Each mosquito bites humans at constant rate \(\beta \).
-
When an uninfected mosquito bites a blood-stage infected human (that is, a human with at least one ongoing relapse or primary infection), human-to-mosquito transmission occurs with probability q.
-
When an infected mosquito bites any human in the population, mosquito-to-human transmission occurs with probability p. This involves the establishment of:
-
a primary (blood-stage) infection, which increases the MOB of the human host by one; and
-
a batch of hypnozoites in the liver, where the batch sizes are geometrically-distributed with PMF (1) and are i.i.d. across bites.
-
-
Within a human, each hypnozoite and primary infection is governed by an independent stochastic processes.
-
Within a human, each hypnozoite undergoes activation at rate \(\alpha \) and death at rate \(\mu \).
-
Within a human, each primary infection or relapse (triggered by a hypnozoite activation event) is cleared independently at constant rate \(\gamma \).
-
Each infected mosquito is replaced by an uninfected mosquito at rate g.
We can formulate the state space of the Markov chain in several ways. One approach is to label each human with a hypnozoite/MOB state, and each mosquito with a binary infection state. Specifically, we can denote the state at time t as \(({\textbf{u}}(t), {\textbf{v}}(t))\) where
-
\({\textbf{u}}(t)\) is a \(P_H\)-dimensional vector whose \(r^{th}\) component itself is a vector giving the number of ‘individuals’ in compartment H, and the sum of individuals in compartments A and P in Fig. 1 for the \(r^{th}\) human; and
-
\({\textbf{v}}(t)\) is a is a \(P_M\)-dimensional vector whose \(s^{th}\) component is 1 if the \(s^{th}\) mosquito is infected and zero otherwise,
yielding the state space
We adopt this formulation of the state space to construct a coupling argument, relative to an ensemble of independent queueing networks, that allows us to characterise the steady state behaviour of the Markov chain (see the proof of Theorem 3.1).
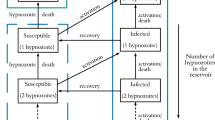
Alternatively, we can formulate the state space to count the number of humans and mosquitoes in each respective infection state. At time t, define
-
\(h_{i,j}(t)\), \(i,j \in \mathbbm {Z}_{\ge 0}\) to be the number of humans with a hypnozoite reservoir of size i and MOB precisely j, and
-
\(m_i(t)\), \(i \in \{0, 1 \}\) to be the number of uninfected and infected mosquitoes respectively.
We can thus denote the state of the Markov chain at time t to be \(( {\textbf{h}}(t), {\textbf{m}}(t))\) where
The state space is then
where the \(\big ( \frac{1}{2}(i+j+1)(i+j) + i +1\big )^{\text {th}}\) term of \({\textbf{h}}(t)\) corresponds to \(h_{i,j}(t)\). For notational convenience, we denote by \(\mathbf {e_{i,j}}\) the \(\big ( \frac{1}{2}(i+j+1)(i+j) + i +1\big )^{\text {th}}\) (unit) coordinate vector in \(\mathbbm {R}^{\textbf{N}}\).
We proceed with the state description \(({\textbf{h}}(t), {\textbf{m}}(t))\) to obtain a density-dependent Markov population process, for which we can recover a FLLN using the work of Barbour and Luczak (2012) (Theorem 3.2). The transition rates are
which can be understood as follows:
-
\(q_{ ( {\textbf{h}}, {\textbf{m}}), ( {\textbf{h}} - \mathbf {e_{i, j}} + \mathbf {e_{i, j-1}}, {\textbf{m}})}\): a human with hypnozoite reservoir size i and MOB j clears a single brood.
-
\(q_{ ( {\textbf{h}}, {\textbf{m}}), ( {\textbf{h}} - \mathbf {e_{i, j}} + \mathbf {e_{i-1, j}}, {\textbf{m}})}\): a single hypnozoite dies in a human with hypnozoite reservoir size i and MOB j.
-
\(q_{ ( {\textbf{h}}, {\textbf{m}}), ( {\textbf{h}} - \mathbf {e_{i, j}} + \mathbf {e_{i-1, j+1}}, {\textbf{m}})}\): a single hypnozoite activates, thereby giving rise to a relapse (that is, a blood-stage infection with one additional brood), in a human with hypnozoite reservoir size i and MOB j.
-
\(q_{ ( {\textbf{h}}, {\textbf{m}}), ( {\textbf{h}} - \mathbf {e_{i, j}} + \mathbf {e_{i + k, j+1}}, {\textbf{m}})}\): a human with hypnozoite reservoir size i and MOB j gains an additional batch of k hypnozoites, in addition to a primary infection (that is, a blood-stage infection with one additional brood) through the bite of an infected mosquito.
-
\(q_{( {\textbf{h}}, {\textbf{m}}), ( {\textbf{h}}, {\textbf{m}} - \mathbf {e_1} + \mathbf {e_{0}})}\): an infected mosquito dies to give rise to uninfected progeny.
-
\(q_{( {\textbf{h}}, {\textbf{m}}), ( {\textbf{h}}, {\textbf{m}} - \mathbf {e_0} + \mathbf {e_{1}})}\): an uninfected mosquito is infected by taking a bloodmeal from an infected human.
We set the ratio of the human and mosquito population sizes to be \(v:= \frac{P_M}{P_H}\), allowing us to write the transition rates given by Eqs. (7) to (12) in the form
where \(\omega _{J_h, J_m}: {\mathcal {R}} \rightarrow \mathbbm {R}\) are given by
As such, we have defined a density-dependent Markov process with size parameter \(P_M\).
3.1 Steady state behaviour
The disease-free state is necessarily absorbing. However, since we have a density-dependent Markov population process with countably many types, absorption in the disease-free state is not guaranteed a priori since the mean MOB and/or hypnozoite reservoir size in the population can drift to infinity (Luchsinger 2001).
In Theorem 3.1 below, we show that the process does, in fact, reach the disease free state with probability one. To do this, we adopt the state description \(({\textbf{u}}(t), {\textbf{v}}(t))\) whereby each human is labelled with a hypnozoite/MOB state, and each mosquito is assigned a binary infection state.
If the arrivals of blood stage infections and batches of hypnozoites to the humans and infections to the mosquitoes were independent Poisson processes, then the process \(({\textbf{u}}(t),{\textbf{v}}(t))\) could be regarded as modelling \(P_H\) independent batch arrival infinite server queueing processes with a structure as in Fig. 1 and \(P_M\) independent on-off processes whose \(s^{th}\) component tells us whether the sth mosquito is infected or not.
However this is not quite the case. An ‘arrival’ of an infection to a human depends on the number of infected mosquitoes and conversely, an ‘arrival’ of an infection to a mosquito depends on the number of infected humans.
To overcome this, we couple the actual process \(({\textbf{u}}(t),{\textbf{v}}(t))\) with a second process \(({\textbf{u}}'(t),{\textbf{v}}'(t))\) in which humans (mosquitoes) become infected at constant rates independent of the number of mosquitoes (humans) that are infected. Specifically, in the process \(({\textbf{u}}'(t),{\textbf{v}}'(t))\), in modelling the rate at which humans become infected, we assume that all the mosquitoes are infected all of the time and, in modelling the rate at which mosquitoes become infected, we assume that all the humans are infected all of the time. This amount of infection in the process \(({\textbf{u}}'(t),{\textbf{v}}'(t))\) can be shown to dominate the amount of infection in \(({\textbf{u}}(t),{\textbf{v}}(t))\). Furthermore, we can recognise \(({\textbf{u}}'(t),{\textbf{v}}'(t))\) as an independent network of infinite server queues for which a stability criterion is known, and hence establish that this criterion must apply to \(({\textbf{u}}(t),{\textbf{v}}(t))\) as well.
The details are given below.
Theorem 3.1
With probability one, disease is eventually eliminated, that is,
and the time to disease elimination has finite expectation.
Proof
To show that absorption in the disease-free state \(({\textbf{u}}(t), {\textbf{v}}(t))=({\textbf{0}}, {\textbf{0}}\)) occurs almost surely, with a finite expected time to absorption, we adopt a coupling argument.
Consider an ensemble of \(P_H\) independent networks of infinite server queues, as defined in Sect. 2, each with homogeneous arrival rate \(\beta p P_M/P_H\). Define the random vector \({{\textbf {u{'}}}}(t) \in ({\mathbbm {N}} \times {\mathbbm {N}})^{P_H}\) to be the vector whose rth component is a vector encoding the numbers of individuals in compartment H, and the sum of individuals in compartments A and P of network or ‘human’ r at time t. Further, consider \(P_M\) independent continuous-time Markov chains, each with state space \(\{ 0, 1\}\) and transition rate matrix
and let the vector \(\mathbf {v'}(t) \in \{0, 1\}^{P_M}\) denote the state of each chain or ‘mosquito’ \(s=1, \dots , P_M\) at time t.
We generate a coupling of the processes \(\{({\textbf{u}}(t), {\textbf{v}}(t)): t \ge 0 \}\) and \(\{({\textbf{u}}'(t), {\textbf{v}}'(t)): t \ge 0 \}\) as follows. Consider \(P_M \times P_H\) independent homogeneous Poisson processes \(\{ B_{rs}(t): t \ge 0 \}\), each of rate \(\beta (p+q)/P_H\). We take \(B_{rs}(t)\) to govern the sequence of interaction times between human r and mosquito s under both processes \(\{({\textbf{u}}(t), {\textbf{v}}(t)): t \ge 0 \}\) and \(\{(\mathbf {u'}(t), \mathbf {v'}(t)): t \ge 0 \}\). The consequences of each human/mosquito interaction, however, can vary:
-
With probability \(p/(p+q)\), a point in the Poisson process \(B_{rs}(t)\) models a potential transmission event from mosquito s to human r. If mosquito s is infected at time t in the process \(\{({\textbf{u}}(t), {\textbf{v}}(t)): t \ge 0 \}\), that is, \(v_s(t) = 1\), then there is a coincident mosquito-to-human transmission event to human r across both processes \(\{({\textbf{u}}(t), {\textbf{v}}(t)): t \ge 0 \}\) and \(\{(\mathbf {u'}(t), \mathbf {v'}(t)): t \ge 0 \}\), with an equal hypnozoite batch size and an identical time course for each inoculated hypnozoite/primary infection. If, in contrast, \(v_s(t) = 0\), then there is an arrival of a geometrically-distributed hypnozoite batch and a single primary infection (with an associated time course) into human r under the process \(\{(\mathbf {u'}(t), \mathbf {v'}(t)): t \ge 0 \}\) only.
-
Otherwise, the point in the Poisson process \(B_{rs}(t)\) models a potential transmission event from human r to mosquito s. If human r is blood-stage infected at time t in the process \(\{({\textbf{u}}(t), {\textbf{v}}(t)): t \ge 0 \}\), that is, \((u_r)_2(t) \ge 1\), then mosquito s immediately enters the infected state, where it remains for a common exponentially-distributed period of mean length 1/g under both processes \(\{({\textbf{u}}(t), {\textbf{v}}(t)): t \ge 0 \}\) and \(\{(\mathbf {u'}(t), \mathbf {v'}(t)): t \ge 0 \}\). If, in contrast, \((u_r)_2(t) = 0\), then mosquito s enters the infected state in the process \(\{(\mathbf {u'}(t), \mathbf {v'}(t)): t \ge 0 \}\) only.
Under this setting, we necessarily have
In particular,
and as such, the hitting time
yields an upper bound for the time to absorption in the disease-free state under the process \(\{({\textbf{u}}(t), {\textbf{v}}(t)): t \ge 0 \}\) with initial condition \(({\textbf{u}}(0), {\textbf{v}}(0)) = (\mathbf {u_0}, \mathbf {v_0})\).
Using Corollary 4.1.1 of Mehra and Taylor (2023), since the expected network occupation time for each hypnozoite/primary infection is finite and the hypnozoite batch size has finite mean, each component \(\{ u'_r(t): t \ge 0 \}\), \(r=1, \dots , P_H\) is ergodic. Further, since each Markov chain \(\{ v'_s(t): t \ge 0 \}\), \(s=1, \dots , P_M\) is irreducible and possesses a finite state space, it is also ergodic.
Each component \(\{ u'_r(t): t \ge 0 \}\) and \(\{ v'_s(t): t \ge 0 \}\) is positive recurrent and thus possesses a stationary distribution. The components evolve independently, therefore the stationary distribution for the multidimensional product \(\{ (\mathbf {u'}(t), \mathbf {v'}(t)): t \ge 0\}\) should be given by the product of stationary distributions of each individual component. Since \(\{ (\mathbf {u'}(t), \mathbf {v'}(t)): t \ge 0\}\) is non-explosive, it follows that it must be positive recurrent. This establishes that each state \((\mathbf {u_0}, \mathbf {v_0}) \in \chi '\) is positive recurrent and the return time to the disease-free state has finite expectation \({{\,\mathrm{\mathbbm {E}}\,}}[T_{({\textbf{0}}, {\textbf{0}})}] < \infty \).
Any state \((\mathbf {u_0}, \mathbf {v_0}) \in \chi '\) can be reached from the disease-free state \(({\textbf{0}}, {\textbf{0}})\) with positive probability \(p(\mathbf {u_0}, \mathbf {v_0}) > 0\) prior to return to the disease-free state through a concerted series of mosquito-inoculation events, with appropriate constraints on the time course of each infection and mosquito lifetimes. Consequently,
that is, the expected hitting time for the disease-free state under the process \(\{ (\mathbf {u'}(t), \mathbf {v'}(t)): t \ge 0\}\) has finite mean, irrespective of the initial condition \((\mathbf {u_0}, \mathbf {v_0}) \in \chi '\).
Since the time to absorption in the disease free state under the process \(\{ ({\textbf{u}}(t), {\textbf{v}}(t)): t \ge 0\}\) with intial condition \((\mathbf {u_0}, \mathbf {v_0}) \in \chi '\) is bounded above by \(T_{(\mathbf {u_0}, \mathbf {v_0})}\), it immediately follows that
and that the time to disease elimination, moreover, has finite expectation. \(\square \)
3.2 A functional law of large numbers
We now use the work of Barbour and Luczak (2012) to obtain a FLLN for the density-dependent Markov population process. In Theorem 3.2 below, we show that the sample paths of the continuous time Markov chain converge to the solution of
in the limit \(P_M \rightarrow \infty \). The mode of convergence, and the relevant space of convergence, are made explicit in Theorem 3.2.
Here, we define the \(\eta \)-norm
and the set
To show that the the semilinear problem (19) with initial condition \(({\textbf{H}}(0), {\textbf{M}}(0)) \in {\mathcal {R}}_\eta \) has a unique mild solution in the interval \([0,\infty )\), we use the results of Barbour and Luczak (2012) (which draw on Theorem 1.4, Chapter 6 of Pazy (2012)), bounding the \(\eta \)-norm of \(( {\textbf{H}}, {\textbf{M}} )\) using the expected occupancy of nodes H, A and P of the network introduced in Sect. 2 (Appendix A.2). To show the convergence of sample paths to the semilinear problem (19), we use Theorem 4.7 of Barbour and Luczak (2012) after verifying a series of semigroup and transition rate assumptions (Appendices A.1 and A.3).
Theorem 3.2
The semilinear problem (19) with initial condition \(({\textbf{H}}(0), {\textbf{M}}(0)) \in {\mathcal {R}}_\eta \) has a unique mild solution in the interval \([0,\infty )\). For each \(T>0\), \(\exists K_T^{(1)}, K_T^{(2)}, K_T^{(3)}\) such that if
for \(P_M\) large enough, then
4 A deterministic population-level model
We devote the present section to the analysis of the semilinear system given by Eq. (19), which arises as the FLLN limit (Barbour and Luczak 2012) for the density-dependent Markov population process detailed in Sect. 3.
4.1 A countable system of ODEs
It is instructive to write out the components of the vector equation (19), rescaled to consider the proportion of humans in each hypnozoite/MOB state. At time t, define
-
\(H_{i,j}(t)\) to be the proportion of humans with hypnozoite reservoir size i and MOB j;
-
\(I_M(t)\) to be the proportion of infected mosquitoes.
Then we obtain the the infinite-dimensional system of ODEs
where, for notational convenience, we have set
The case \(\nu =0\) yields the classical model of superinfection for P. falciparum, as formulated by Bailey (1957) (Appendix E).
Equation (20) constitutes a deterministic compartmental model for a vector that has total ‘mass’ one. If we ignore the coupling to Eq. (21) and think of (20) as a system of equations driven by the time-varying function \(I_M(t)\), we observe that they have a form identical to the Kolmogorov forward differential equations for a continuous-time Markov chain model with state space \(\mathbbm {Z}^2\), where \(H_{i,j}(t)\) has the interpretation that the Markov chain is in state (i, j) at time t and \(aI_M(t)\) is the time-varying input of a Poisson process. This model is precisely the open network of infinite server queues governing within-host superinfection and hypnozoite dynamics that we discussed in Sect. 2, and Eq. (20) is indeed the forward Kolmogorov differential equation for this Markov chain.
In the population model, the process of mosquito-to-human transmission is not Poisson because of coupling between the human and mosquito populations. However, since the host part of the population model has an identical form to the Kolmogorov forward differential equations of the queueing network, it must have the same time-dependent generating function. Rather than solving the system of ODEs (20) directly for some time dependent function \(I_M(t)\), we can draw on a physical understanding of the queueing network to derive a generating function for the time dependent state probabilites \(H_{i,j}(t)\) as a function of \(I_M(\tau )\), \(\tau \in [0, t)\). In particular, from Eq. (6), given the absence of liver- and blood-stage infection in the human population at time zero, that is,
it follows that
where Eq. (22) is guaranteed to converge in the domain \((x, y) \in [0, 1]^2\) for any fixed t.
We can exploit the functional dependence (22) between the generating function for the state probabilities \(H_{i,j}(t)\) of the human population at time t, and the proportion of infected mosquitoes \(I_M(\tau )\) for \(\tau \in [0, t)\) to aid analysis of the infinite-compartment model given by Eqs. (20) and (21).
4.2 A reduced integrodifferential equation
Upon examination of the deterministic model structure, we note that the coupling between human and mosquito dynamics is completely determined by the time evolution of
-
the prevalence of blood-stage infection in the human population (that is, humans with MOB at least one), which can be written
$$\begin{aligned} I_H(t) := \sum ^\infty _{i=0} \sum ^\infty _{j=1} H_{i, j}(t); \end{aligned}$$ -
the FORI \(a I_M(t)\).
The utility of our observation in Sect. 4.1 emerges in the characterisation of the functional dependence between \(I_H(t)\) and the FORI \(a I_M(\tau )\) for \(\tau \in [0, t)\). Specifically, given
we can readily write
using the generating function (22).
Substituting the integral equation (24) into the ODE (21) yields the IDE
When the human population is initially blood- and liver-stage infection-naive (that is, initial condition (23) holds), the time evolution of the FORI \(aI_M(t)\) is equivalent under the IDE (25), and the infinite-dimensional system of ODEs given by Eqs. (20) and (21).
As a function of the FORI \(a I_M(\tau )\), \(\tau \in [0, t)\) which solves the IDE (25), we can use the generating function (22) to recover the population-level distribution of hypnozoite and superinfection states in the human population at time t. The recurrence relations provided in Mehra and Taylor (2023) can be used to obtain the proportion of humans \(H_{i,j}(t)\) in each hypnozoite/MOB state. The time-evolution of various quantities of biological interest can be recovered using the formulae provided in Mehra et al. (2022).
4.3 The steady state distribution
Steady state analysis for the countably infinite system of ODEs given by Eqs. (20) and (21) is not straightforward. Instead, we characterise the existence and asymptomatic stability of equilibria for the IDE (25).
Upon inspection, we find that an equilibrium solution \(I_M^*\) of the IDE (25) must satisfy the integral equation
The disease-free equilibrium \(I_M^*=0\) clearly satisfies Eq. (26). On the domain \(I_M^* \in [0, 1]\), we note that the LHS of (26) is a monotonically increasing, convex function of \(I_M^*\) that approaches infinity in the limit \(I_M^* \rightarrow 1\) while the RHS is monotonically increasing, concave. The existence of a second solution to Eq. (26) therefore depends on the limiting slope as \(I_M \rightarrow 0\). It exists if and only if the derivative of the LHS is less than the derivative of the RHS at \(I_M^*=0\) (see Fig. 2).
Schematic of the geometric argument to establish the existence of a non-zero solution to Eq. (26)
This allows us to identify a bifurcation parameter
with \(R_0>1\) a necessary and sufficient condition for the existence of an endemic equilibrium \(I_M^*>0\).
We can readily interpret the integral expression in Eq. (27). The probability of a current blood-stage infection time \(\tau \) after an infective bite is given by the integrand
The integral
therefore yields the expected cumulative duration of blood-stage infection attributable to a single infective bite. The functional form of the bifurcation parameter \(R_0\) is analogous to that of a reproduction number (Diekmann et al. 1990).
Using the stability criterion of Brauer (1978), whereby the IDE (25) is linearised about each equilibrium solution, we can also establish sufficient conditions for equilibrium solutions \(I_M^*\) to be uniformly asymptotically stable. The threshold behaviour of the IDE (25) is summarised in Theorem 4.1 below, with a proof provided in Appendix B.
Theorem 4.1
(Threshold behaviour for the IDE (25))
-
1.
If \(R_0 < 1\), then the disease-free equilibrium \(I_M^* = 0\) is uniformly asymptotically stable and no endemic equilibrium solution exists.
-
2.
If \(R_0 > 1\), there exists precisely one endemic equilibrium \(I_M^* >0 \), given by the non-trivial solution to the equation
$$\begin{aligned} 1 - \frac{g I_M^*}{b(1-I_M^*)} - e^{-\frac{g}{b} R_0^2 I_M^*} = 0 \end{aligned}$$(28)This endemic equilibrium \(I^*_M>0\) is uniformly asymptotically stable if
$$\begin{aligned} I_M^* > \frac{1 + \frac{b}{b+g} - \sqrt{\big ( 1 - \frac{b}{b+g} \big )^2 + 4 \frac{b}{b+g} \frac{1}{R_0^2} }}{2}. \end{aligned}$$
5 A general strategy for constructing tractable models of malarial superinfection
Our conceptual approach for modelling superinfection and hypnozoite dynamics for vivax malaria has been three-fold.
To establish a functional relationship between the intensity of mosquito-to-human transmission, and the dynamics of superinfection and hypnozoite accrual on the within-host scale (Sect. 2), we construct an open network of infinite server queues that accounts for stochasticity in:
-
the temporal sequence of mosquito-to-human transmission events, modelled with a non-homogeneous Poisson process;
-
parasite inocula and consequently hypnozoite batch sizes, assumed to be geometrically-distributed for each bite (White et al. 2014); and
-
the time course of each hypnozoite and primary infection, governed by independent stochastic processes.
The independence structure of the queueing network enables us to characterise the occupancy distribution through relatively straightforward physical arguments (Mehra et al. 2022).
We model population level dynamics by constructing a Markov population process to address the dependence between the burden of blood-stage infection in the human population, and the intensity of mosquito-to-human transmission (Sect. 3). To characterise the steady state behaviour, we couple the Markov population process to an independent ensemble of queueing networks, with the same structure as the within host model and a known stability criterion (Theorem 3.1). Re-formulation of the state space to count the number of humans and mosquitoes in each permissible infection state, followed by a rescaling argument, yields a density-dependent Markov population process for which a FLLN limit, taking the form of a countably-infinite system of ODEs, is recovered using the work of Barbour and Luczak (2012) (Theorem 3.2).
We pay particular attention to the FLLN limit, recognising that it takes a form identical to what could be derived using a deterministic compartmental model of the type that are ubiquitous in mathematical epidemiology (Sect. 4). By recognising that those parts of the compartmental model that relate to the infection status of the human population are identical to the Kolmogorov forward differential equations for a network of infinite-server queues, we propose, to the best of our knowledge, a novel reduction to collapse the countable system of ODEs into a reduced IDE that is more amenable to analysis, and for which a threshold phenomenon can be characterised (Theorem 4.1).
A common vein in the analysis of population-level models through the course of this manuscript is the utility of a physical understanding of the underlying within-host model. We now seek to illustrate how the ideas presented in this manuscript can be adopted more generally to construct tractable models of malarial superinfection, with appropriate constraints on the model structure.
5.1 From a countable system of ODEs to a reduced integrodifferential equation
We arrived at the countable system of ODEs given by Eqs. (20) and (21) by recovering a FLLN for an appropriately-scaled density dependent Markov population process. Following a standard compartment modelling approach, however, we can view this system as a natural extension of the Ross-Macdonald framework to allow for superinfection (Bailey 1957) and hypnozoite accrual (White et al. 2014).
We can also derive Eqs. (20) and (21) under the “hybrid approximation”, as delineated by Naasell (2013); Henry (2020). Equation (20), which captures the time evolution of the within-host PMF, can also be interpreted as the governing equation for the expected frequency distribution in the human population, as a function of the FORI. Similarly, Eq. (21) is precisely the Kolmogorov forward differential equation governing the time evolution of the probability that each mosquito in the population is infected—which can likewise be interpreted as the expected proportion of infected mosquitoes—as a function of the prevalence of blood-stage infection in the human population. The premise of the “hybrid approximation” is to allow for stochasticity within the human and mosquito populations respectively, but to couple host and vector dynamics through expected values (Henry 2020; Naasell 2013); the hybrid approximation to the present model of hypnozoite accrual and superinfection yields precisely Eqs. (20) and (21). Parallels between the FLLN and hybrid approximation have been noted previously in the literature: according to Hadeler and Dietz (1983), Rost (1979) proves that hybrid and mean-field approximations are equivalent,Footnote 1 while Lewis (1975) establishes similar results through simulation.
Irrespective of the derivation of the infinite-compartment model it is the observation that Eq. (20) constitutes the Kolmogorov forward differential equations for an open network of infinite server queues (Sect. 4.1) that constitutes the crux of our analysis. Rather than solving the system of ODEs (20) directly, we use a physical understanding of the queueing structure to derive the PGF (22). From the PGF, we extract the prevalence of blood-stage infection as a function of the FORI—which underpins the coupling between host and vector dynamics, and enables the derivation of a single IDE governing the FORI; in addition to quantities of biological interest, the time evolution of which can be recovered as a function of the FORI solving the aforementioned IDE.
Challenges posed by infinite-dimensional systems of ODEs for simulation and analysis have prompted the development of methods like the pseudoequilibrium approximation to yield finite-dimensional systems of ODEs in the presence of superinfection (Dietz et al. 1974; Henry 2020). Our IDE reduction offers an alternative construction that yields an identical FORI to the original system of ODEs, under the assumption that the human population is initially uninfected.
Relative to the countable system of ODEs which served as our starting point, the reduced IDE is more amenable to steady state analysis. For compartment models with finitely many types (comprising finite-dimensional systems of ODEs), the next generation matrix method of Diekmann et al. (2010) is routinely used to characterise threshold phenomena: the basic reproduction number \(R_0\)—which quantifies the number of additional cases stemming from an index case—is computed as the spectral radius of a “next generation operator”, with the key implication that \(R_0 < 1\) is a necessary and sufficient condition for the stability of the trivial (disease-free) equilibrium (Heffernan et al. 2005). While the notion of a reproduction number and spectral bound generalises to epidemic models with countably many types (Thieme 2009), computation is not straightforward. In the present manuscript, we apply the stability criterion of Brauer (1978) to perform an asymptotic stability analysis for the reduced IDE, but are unaware of readily-verifiable stability criteria applicable to the countable system of ODEs.
5.2 Analyticity at the within-host scale
The strategy delineated above is underpinned by the fact that the within-host model is analytically-tractable. The critical assumption is that consequences of each mosquito-to-human transmission event are governed by independent stochastic processes, for which a time-dependent PGF can be derived. We can accommodate multiple ‘types’ of incoming parasites/infections (here, we distinguish hypnozoites from immediately developing parasite forms, represented by a primary infection), and occupancy times for each parasite/lifecycle stage do not necessarily have to be Markovian (that is, we can allow for general service time distributions). Tractable extensions to the within-host model that permit the IDE reduction proposed here encompass the class of models analysed in Mehra and Taylor (2023), comprising open networks of infinite server queues with nonhomogeneous multivariate batch Poisson arrivals. They are applicable when the evolution of ‘individuals’ (hypnozoites or blood-stage infections in this case) occurs independently. Importantly, this is not the case when there is competition or another form of interaction between parasite broods. Further, while service time distributions and arrival rates can vary deterministically with time, they should not be dependent on the within-host state, barring the consideration of certain forms of immunity.
Nonetheless, our work provides a theoretical basis to explore the epidemiology of malarial superinfection, with natural extensions to allow for greater biological realism. A generalisation of the present framework, allowing for long-latency hypnozoites (White et al. 2014; Mehra et al. 2020); time-varying transmission parameters, and the acquisition of transmission-blocking and antidisease immunity for P. vivax, is presented in Mehra et al. (2022). There, we derive an infinite compartment model under a “hybrid approximation” (Henry 2020; Naasell 2013), comprising an infinite-dimensional system of ODEs, from which we derive a reduced system of IDEs using the approach detailed in Sect. 4. Analyses of both steady state solutions and transient dynamics under this generalised deterministic model are tractable using the within-host distributions derived in Mehra et al. (2022).
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Notes
We have only been able to find the citation for Rost (1979), but not the actual conference paper.
References
Aguas R, Ferreira Marcelo U, Gomes M, Gabriela M (2012) Modeling the effects of relapse in the transmission dynamics of malaria parasites. J Parasitol Res
Alonso D, Dobson A, Pascual M (2019) Critical transitions in malaria transmission models are consistently generated by superinfection. Philos Trans R Soc B 374(1775):20180275
Anwar MN, Hickson RI, Mehra S, Price DJ, McCaw JM, Flegg MB, Flegg JA (2023) Optimal interruption of P. vivax malaria transmission using mass drug administration. Bull Math Biol 85(6):43
Bailey Norman TJ (1957) The mathematical theory of epidemics. Charles Griffin & Company Limited
Barbour AD, Luczak MJ (2012) A law of large numbers approximation for Markov population processes with countably many types. Probab Theory Relat Fields 153(3):727–757
Battle KE, Kevin Baird J (2021) The global burden of Plasmodium vivax malaria is obscure and insidious. PLoS Med 18(10):e1003799
Boucherie RJ, Taylor PG (1993) Transient product from distributions in queueing networks. Discrete Event Dyn Syst 3(4):375–396
Brauer F (1978) Asymptotic stability of a class of integro-differential equations. J Differ Equ 28(2):180–188
Chamchod F, Beier JC (2013) Modeling Plasmodium vivax: relapses, treatment, seasonality, and G6PD deficiency. J Theor Biol 316:25–34
Commons RJ, Simpson JA, Watson J, White NJ, Price RN (2020) Estimating the proportion of Plasmodium vivax recurrences caused by relapse: a systematic review and meta-analysis. Am J Trop Med Hygiene 103(3):1094
Diekmann O, Heesterbeek JAP, Metz JAJ (1990) On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J Math Biol 28:365–382
Diekmann O, Heesterbeek JAP, Roberts MG (2010) The construction of nextgeneration matrices for compartmental epidemic models. J R Soc Interface 7(47):873–885. https://doi.org/10.1098/rsif.2009.0386
Dietz K, Molineaux L, Thomas A (1974) A malaria model tested in the African savannah. Bull World Health Org 50(3–4):347
Eick SG, Massey WA, Whitt W (1993) The physics of the \({{\rm Mt/G}}/\infty \) queue. Oper Res 41(4):731–742
Gemperli A, Vounatsou P, Sogoba N, Smith T (2006) Malaria mapping using transmission models: application to survey data from Mali. Am J Epidemiol 163(3):289–297
Hadeler KP, Dietz K (1983) Nonlinear hyperbolic partial differential equations for the dynamics of parasite populations. In: Hyperbolic partial differential equations. Elsevier, pp 415–430
Heffernan JM, Smith RJ, Wahl LM (2005) Perspectives on the basic reproductive ratio. J R Soc Interface 2(4):281–293
Henry JM (2020) A hybrid model for the effects of treatment and demography on malaria superinfection. J Theor Biol 491:110194
Ishikawa H, Ishii A, Nagai N, Ohmae H, Harada M, Suguri S, Leafasia J (2003) A mathematical model for the transmission of Plasmodium vivax malaria. Parasitol Int 52(1):81–93
Lewis T (1975) A model for the parasitic disease bilharziasis. Adv Appl Probab 7(4):673–704
Luchsinger CJ (2001) Stochastic models of a parasitic infection, exhibiting three basic reproduction ratios. J Math Biol 42(6):532–554
Macdonald G (1950) The analysis of infection rates in diseases in which superinfection occurs. Trop Dis Bull 47:907–915
Mehra S (2022) Epidemic models for malaria: superinfection. MA thesis. School of Mathematics and Statistics, University of Melbourne
Mehra S, McCaw JM, Flegg MB, Taylor PG, Flegg JA (2020) An activation-clearance model for Plasmodium vivax malaria. Bull Math Biol 82(2):32
Mehra S, McCaw JM, Flegg MB, Taylor PG, Flegg JA (2021) Antibody dynamics for Plasmodium vivax malaria: a mathematical model. Bull Math Biol 83(1):1–27
Mehra S, Stadler E, Khoury D, McCaw JM, Flegg JA (2022) Hypnozoite dynamics for Plasmodium vivax malaria: the epidemiological effects of radical cure. J Theor Biol 111014
Mehra S, Taylor PG (2023) Open networks of infinite server queues with nonhomogeneous multivariate batch Poisson arrivals. Queueing Syst. https://doi.org/10.1007/s11134-023-09891-x
Mehra S, Taylor PG, McCaw JM, Flegg JA (2022) A hybrid transmission model for Plasmodium vivax accounting for superinfection, immunity and the hypnozoite reservoir. arXiv:2208.10403
Mikolajczak SA et al (2015) Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host Microbe 17(4):526–535
Mueller I, Galinski MR, Kevin Baird J, Carlton JM, Kochar DK, Alonso PL, del Portillo HA (2009) Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Diseases 9(9):555–566
Nåsell I (2013) Hybrid models of tropical infections, vol 59. Springer
Nåsell I (1991) On the quasi-stationary distribution of the Ross malaria model. Math Biosci 107(2):187–207
Nedelman J (1984) Inoculation and recovery rates in the malaria model of Dietz, Molineaux, and Thomas. Math Biosci 69(2):209–233
Nurul Anwar Md, Hickson Roslyn I, Mehra Somya, McCaw James M, Flegg Jennifer A (2022) A multiscale mathematical model of Plasmodium vivax transmission. Bull Math Biol 84(8):81
Pazy Amnon (2012) Semigroups of linear operators and applications to partial differential equations, vol 44. Springer
Popovici Jean et al (2018) Genomic analyses reveal the common occurrence and complexity of Plasmodium vivax relapses in Cambodia. In: MBio 9.1
Reto Hagmann J, Charlwood D, Gil V, Ferreira C, Rosário VD, Smith TA (2003) Malaria and its possible control on the island of Príncipe. Malaria J 2(1):1–9
Robinson LJ et al (2015) Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med 12(10):e1001891
Rost H (1979) On the method of hybrid model approximation. Univ., Sonderforschungsbereich 123
Roy M, Bouma MJ, Ionides EL, Dhiman RC, Pascual M (2013) The potential elimination of Plasmodium vivax malaria by relapse treatment: insights from a transmission model and surveillance data from NW India. PLoS Neglect Trop Dis 7(1):e1979
Schäfer C, Zanghi G, Vaughan AM, Kappe SHI (2021) Plasmodium vivax latent liver stage infection and relapse: biological insights and new experimental tools. Annu Rev Microbiol 75:87–106
Smith DL, Hay SI (2009) Endemicity response timelines for Plasmodium falciparum elimination. Malaria J 8(1):1–13
Smith DL, Guerra CA, Snow RW, Hay SI (2007) Standardizing estimates of the Plasmodium falciparum parasite rate. Malaria J 6(1):1–10
Thieme HR (2009) Spectral bound and reproduction number for infinite-dimensional population structure and time heterogeneity. SIAM J Appl Math 70(1):188–211
Venugopal K, Hentzschel F, Gediminas V, Matthias M (2020) Plasmodium asexual growth and sexual development in the haematopoietic niche of the host. Nat Rev Microbiol 18(3):177–189
White MT, Karl S, Battle KE, Hay SI, Mueller I, Ghani AC (2014) Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission. eLife 3:e04692
White MT et al (2018) Mathematical modelling of the impact of expanding levels of malaria control interventions on Plasmodium vivax. Nat Commun 9(1):1–10
White NJ, Imwong M (2012) Relapse. Adv Parasitol 80:113–150
White MT, Shirreff G, Karl S, Ghani AC, Mueller I (2016) Variation in relapse frequency and the transmission potential of Plasmodium vivax malaria. Proc R Soc B: Biol Sci 283(1827):20160048
WHO. World Malaria Report (2021) World Health Organization. Switzerland, Geneva, p 2021
William A, Whitt Massey Ward (1993) Networks of infinite-server queues with nonstationary Poisson input. Queueing Syst 13(1):183–250
Willmot GE, Drekic S (2001) On the transient analysis of the \({{\rm MX/M}}/\infty \) queue. Oper Res Lett 28(3):137–142
Xekalaki E (1987) A method for obtaining the probability distribution of m components conditional on \(\ell \) components of a random sample. Rev Roumaine Math Pure Appl 32:581–583
Xu A, Cen Z (2011) A unified approach to some recurrence sequences via Faa di Bruno’s formula. Comput Math Appl 62(1):253–260
Acknowledgements
The authors thank Jennifer Flegg for valuable discussions around the incorporation of superinfection in population-level models. JMM acknowledges support from the Australian Research Council through ARC Discovery Project DP210101920. PGT acknowledges support from the Australian Research Council through Laureate Fellowship FL130100039.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
A A functional law of large numbers
We now verify the conditions for the FLLN (Barbour and Luczak 2012) of the Markov population process governing hypnozoite and superinfection dynamics, as described in Sect. 3. The scaled transition rates \(\omega _J\) are given by Eqs. (13) to (18). We define
with each \(({\textbf{H}}, {\textbf{M}}) \in {\mathcal {R}}\) indexed in the form
in addition to the subset
Let \({\mathcal {J}}\) be the set of jumps with non-zero transition rates. Jumps are of “bounded influence” in the sense of Barbour and Luczak (2012), in that at most two compartments are affected. Further,
Therefore, Assumptions 1.2 and 1.3 of Barbour and Luczak (2012) are satisfied, with the implication that Markov process with transition rates given by Eqs. (13) to (18) is a “pure jump process, at least for some non-zero length of time” (Barbour and Luczak 2012).
In the notation of Barbour and Luczak (2012), we write the proposed limit as a semilinear equation
where A is a constant (that is, not dependent on time) matrix, with non-zero terms
while F has components
1.1 A.1 Assumption 2.1 of Barbour and Luczak (2012)
We recapitulate and verify Assumption 2.1 of Barbour and Luczak (2012) below, with changes in notation/exposition in line with the current manuscript.
We begin by introducing the set
By assigning a ‘size’ to each human and mosquito infection state through a fixed vector \(\eta = (\eta ^{(h)}, \eta ^{(m)}) \in {\mathcal {R}}\), such that
we can construct an empirical moment analogue
To bound these empirical moments, Barbour and Luczak (2012) introduce the quantites
In a similar vein, we set
Assumption 2.1 of Barbour and Luczak (2012) then reads as follows.
Assumption 1
There exists \(\eta \in {\mathcal {R}}\) satisfying condition (29) and \(r_\text {max}^{(1)}, r_\text {max}^{(2)} \ge 1\) such that the following conditions hold:
-
1.
For all \(({\textbf{H}}, {\textbf{M}}) \in {\mathcal {X}}_+\)
$$\begin{aligned} W_r \Big ( |{\textbf{M}}|_1^{-1} ({\textbf{H}}, {\textbf{M}}) \Big ) < \infty , \qquad 0 \le r \le r^{(1)}_\text {max}. \end{aligned}$$ -
2.
\(\exists \) non-negative constants \(k_{rl}\) such that, for all \(({\textbf{H}}, {\textbf{M}}) \in {\mathcal {R}}\) with \(|{\textbf{H}}|_1 = 1/v, \, |{\textbf{M}}|_1 = 1\),
$$\begin{aligned} U_0 \big ( ( {\textbf{H}}, {\textbf{M}}) \big )&\le k_{01} S_0 \big ( ( {\textbf{H}}, {\textbf{M}}) \big ) + k_{04}\\ U_1 \big ( ( {\textbf{H}}, {\textbf{M}}) \big )&\le k_{11} S_1 \big ( ( {\textbf{H}}, {\textbf{M}}) \big ) + k_{14}\\ U_r \big ( ( {\textbf{H}}, {\textbf{M}}) \big )&\le \{ k_{r1} + k_{r2} S_0 \big ( ( {\textbf{H}}, {\textbf{M}}) \big ) \} \cdot S_r \big ( ( {\textbf{H}}, {\textbf{M}}) \big ) + k_{r4}, \, 2 \le r \le r^{(1)}_\text {max}. \end{aligned}$$ -
3.
\(\exists \) non-negative constants \(k_{rl}\) and a function p(r) with \(1 \le p(r) \le r_\text {max}^{(1)}\) for \(1 \le r \le r_\text {max}^{(2)}\), such that, for all \(({\textbf{H}}, {\textbf{M}}) \in {\mathcal {R}}\) with \(|{\textbf{H}}|_1 = 1/v, \, |{\textbf{M}}|_1 = 1\)
$$\begin{aligned} V_0 \big ( ( {\textbf{H}}, {\textbf{M}}) \big )&\le k_{03} S_0 \big ( ( {\textbf{H}}, {\textbf{M}}) \big ) + k_{05}\\ V_r \big ( ( {\textbf{H}}, {\textbf{M}}) \big )&\le k_{r3} S_{p(r)} \big ( ( {\textbf{H}}, {\textbf{M}}) \big ) + k_{r5}. \end{aligned}$$
Claim 1
Assumption 1 holds for \(\eta = (\eta ^{(h)}, \eta ^{(m)}) \in {\mathcal {R}}\) with
and arbitrarily large \(r_\text {max}^{(1)}, r_\text {max}^{(2)}\).
Proof
Given our choice of \(\eta \), we can write
We begin by noting that
In the case \(r=1\), we can simplify these expressions substantially to yield the bounds
for all \(({\textbf{H}}, {\textbf{M}} ) \in {\mathcal {R}}\) with \(|{\textbf{H}}|_1 = 1/v, \, |{\textbf{M}}|_1 = 1\).
For \(r \ge 1\) and \(({\textbf{H}}, {\textbf{M}} ) \in {\mathcal {R}}\) with \(|{\textbf{H}}|_1 = 1/v, \, |{\textbf{M}}|_1 = 1\), we obtain the bounds
where \(H_b\) is a geometrically-distributed random variable with mean \(\nu \) and state space \(\mathbbm {Z}_{\ge 0}\), noting that \({{\,\mathrm{\mathbbm {E}}\,}}[H_b^p] < \infty \) for all \(p \ge 0\).
Likewise, for a given value \(({\textbf{H}}, {\textbf{M}}) \in {\mathcal {X}}_+\),
for all \(r > 0\) since the RHS constitutes a finite sum. We thus see that Assumption 1 is satisfied for arbitrarily large \(r_\text {max}^{(1)}, r_\text {max}^{(2)} \ge 1\). \(\square \)
1.2 A.2 Existence of a unique weak solution to the semilinear equation (19)
We now establish the existence and uniqueness of weak solutions to the semilinear equation (19) (which can be written explicitly as the system of ODEs given by Eq. (20) and (21) after re-scaling \(H_{i,j}\) by a factor of v).
We first verify Assumption 3.1 of Barbour and Luczak (2012), by noting that:
We are then required to fix \(\mu \in {\mathcal {R}}\) with \(\mu ^{(h)}_{i,j}, \mu ^{(m)}_k \ge 1\), with the constraint that:
-
1.
there exists \(r \le r_\text {max}^{(2)}\) such that \(\sup _{i, j \ge 0} \{ \mu ^{(h)}_{i,j}/(\eta ^{(h)}_{i,j})^r \} < \infty \) (Assumption 4.2.1 of Barbour and Luczak (2012));
-
2.
\(A^T \mu \le w \mu \) for some \(w \ge 0\) (Assumption 3.2 of Barbour and Luczak (2012)).
Since Assumption 1 is satisfied for arbitrarily large \(r^{(2)}_\text {max}\), it suffices to set \(\mu =\eta \), in which case we can choose \(w = \max \{ \alpha + \mu , \gamma , g\}\). We thus define the \(\eta \)-norm
on the set
The conditions of Theorem 3.1 of Barbour and Luczak (2012) are thus satisfied. The implications of this theorem are as follows. As in Barbour and Luczak (2012), denote by \(P(\cdot )\) “the semigroup of Markov transition matrices corresponding to the minimal process associated with Q”, where
and set
Then R is a strongly continuous semigroup on \({\mathcal {R}}_\eta \), with \(A = R'(0)\) and \(R'(t) = R(t)A\) for all \(t \ge 0\).
It remains to verify Assumption 4.1 of Barbour and Luczak (2012), which mandates that F is locally-Lipschitz in the \(\eta \)-norm. For \(({\textbf{H}}^{(1)}, {\textbf{M}}^{(1)}) \ne ({\textbf{H}}^{(2)}, {\textbf{M}}^{(2)})\) such that
using the inequality
we can show that
where we have interchanged the order of summation and used the geometric summation. Therefore,
whereby Assumption 4.1 of Barbour and Luczak (2012) is satisfied.
Using Theorem 1.4, Chapter 6 of Pazy (2012), Barbour and Luczak (2012) then conclude that the semilinear problem (19) with initial condition \(({\textbf{H}}(0), {\textbf{M}}(0)) \in {\mathcal {R}}_\eta \) has a unique mild solution
on the interval \([0, t_\text {max})\), \(t_\text {max} > 0\), with
To establish a bound on the \(\eta \)-norm of \(({\textbf{H}}(t), {\textbf{M}}(t))\), we draw on Sect. 4.1, recognising Eq. (20) as the Kolmogorov forward differential equation for the network of infinite server queues detailed in Sect. 2. Since the FORI—or equivalently, the infective bite rate experienced by each human—is bounded above by \(a:= \beta p v\), using the generating function given by Eq. (22), we see that for all \(t \ge 0\)
Hence,
1.3 A.3 Assumption 4.2.2 of Barbour and Luczak (2012)
We now verify Assumption 4.2.2 of Barbour and Luczak (2012), reproduced below with modifications in notation/exposition as appropriate.
Assumption 2
There exists \(\zeta \in {\mathcal {R}}\) with \(\zeta ^{(h)}_{i,j}, \zeta ^{(m)}_k \ge 1\) such that
for some \(1 \le r:= r(\zeta ) \le r_\text {max}^{(2)}\) and some \(b=b(\zeta ) \ge 1\), and that
Claim 2
Fix \(v = |{\textbf{M}}|_1/|{\textbf{H}}|_1\). Assumption 2 holds for \(\zeta = (\zeta ^{(h)}, \zeta ^{(m)}) \in {\mathcal {R}}\), such that
with \(b(\zeta )=1\) and \(r(\zeta )=10\).
Proof
For our choice of \(\zeta \in {\mathcal {R}}\), we have
where \(H_b\) is a geometrically-distributed random variable with mean \(\nu \) and state space \(\mathbbm {Z}_{\ge 0}\). Further,
so Assumption 2 is satisfied. \(\square \)
B Proof of Theorem 4.1
Proof
Denote by \(I_M^*\) the steady state FORI. For notational convenience, we set
and note that
From the IDE (25), we deduce that each equilibrium solution \(I_M^*\) necessarily satisfies
The disease free equilibrium \(I_M^* = 0\) clearly satisfies Eq. (32). We begin by characterising the existence of endemic equilibria, following analogous reasoning to Mehra et al. (2022). On the domain \(I_M^* \in [0, 1]\), we observe that:
-
\(f_1(I_M^*):= \frac{g I_M^*}{b(1-I_M^*)}\) is monotonically increasing, convex with \(f_1(I_M^*) \rightarrow \infty \) as \(I_M^* \rightarrow 1\).
-
\(f_2(I_M^*):= 1 - e^{-ac_1 I_M^*}\) is monotonically increasing, concave.
Define \(F(I_M^*):= f_1'(I_M^*)- f_2'(I_M^*)\). Then \(F'(I_M^*) > 0\) for all \(I_M^* \in [0, 1]\), so \(F(I_M^*)\) is monotonically increasing on the interval \(I_M^* \in [0, 1]\) with \(F(I_M^*) \rightarrow \infty \) as \(I_M^* \rightarrow 1\). We thus consider two cases:
-
Case 1: \(F(0) > 0\) Here, it follows that \(F(I_M^*) > 0\) for all \(I_M^* \in [0, 1] \implies (f_1-f_2)\) is monotonically increasing and thus non-zero for all \(I_M^* \in (0, 1]\).
-
Case 2: \(F(0) < 0\) By the intermediate value theorem, there exists \(M_0 \in (0, 1)\) such that \(F(I_M^*)<0\) for all \(I_M^* \in [0, M_0)\) and \(F(I_M^*)>0\) for all \(I_M^* \in (M_0, 1]\). So \((f_1-f_2)\) is monotonically decreasing on the interval \([0, M_0)\) and monotonically increasing on the interval \((M_0, 1)\). Since \((f_1-f_2)(0)=0\) and \((f_1-f_2)(I_M^*) \rightarrow \infty \) as \(I_M^* \rightarrow 1\), it follows there exists unique \(M_1 \in (M_0, 1)\) such that \((f_1-f_2)(M_1)=0\).
Therefore, an endemic equilibrium solution exists iff
and given \(R_0>1\), this endemic equilibrium is unique.
We now characterise the asymptotic stability of the disease-free and endemic equilibria. By Theorem 2 of Brauer (1978), an equilibrium solution \(I_M(t) = I_M^*\) is uniformly asymptotically stable if the trivial solution \(u(t)=0\) to the first-order linear IDE
is uniformly asymptotically stable. By Theorem 1 of Brauer (1978), since \(y(\tau )\) is continuous and non-negative for all \(\tau \in [0, \infty )\), it follows that \(u(t) = 0\) is uniformly asymptotically stable if and only if
Equation (33) serves as a sufficient condition for a steady state solution \(I_M^*\) to be uniformly asymptotically-stable. Noting that any steady state solution \(I_M^*\) must solve Eq. (32), we derive the equivalent condition
Using Eq. (34), it immediately follows that the disease-free equilibrium \(I_M^* = 0\) is uniformly asymptotically stable in the case where \(R_0 < 1\).
When \(R_0>1\), we have \(h(0)>0\) and \(h(1)<0\), so there exists precisely one root \(h(x) =0\) in the domain \(x \in (0, 1)\) and
serves as a sufficient condition for the endemic equilibrium to be uniformly asymptotically stable. \(\square \)
C The pseudeoquilibrium approximation to the superinfection recovery rate proposed by White et al. (2014)
White et al. (2014) construct a deterministic compartmental model, consisting of an infinite-dimensional system of ODEs monitoring the hypnozoite burden and the absence or presence of blood-stage infection. Superinfection is captured through a “pseudoequilibrium approximation” for the rate of recovery from blood-stage infection, conditional on the size of the hypnozoite reservoir. Below, we characterise this construction in detail.
The pseudoequilibrium approximation to the rate of recovery from (super)infection was initially proposed by Dietz et al. (1974). For an \(M/M/\infty \) queue with homogeneous arrival rate \(\lambda \) and service rate \(\gamma \)—which describes within-host superinfection dynamics for P. falciparum in a constant transmission setting—Dietz et al. (1974) note that the expected duration of each busy period, or ‘superinfection’ at steady state is
Therefore, as a function of the FORI \(\lambda (t) = \lambda \) at time t, Dietz et al. (1974) argue that the rate of recovery from superinfection at time t can be approximated to be
This argument essentially replaces a rate of recovery, which necessarily depends on the MOB, with its expectation taken under steady-state conditions with the arrival rate taken to be constant. While this might be a reasonable thing to do if there is no better option, as we show in this paper, the model is still tractable when the MOB is included in the state space. It might be that the pseudoequilibrium approximation yields reasonable results when changes in the FORI occur on a slower time scale than the clearance of blood-stage infection; it has, however, been critiqued on the basis of its inability to capture MOB dynamics following abrupt changes in transmission intensity (Smith and Hay 2009).
To account for superinfection, White et al. (2014) suggest that the recovery rate from blood-stage infection, conditional on the hypnozoite burden i should be
where \(\lambda \) represents the FORI. For an individual with a hypnozoite reservoir of size i, additional blood-stage infections are acquired at rate \(\lambda + i \alpha \)—with primary infections occurring at rate \(\lambda \) due to mosquito inoculation, and each hypnozoite in the liver activating independently at rate \(\alpha \) to give rise to a relapse. Equation (36) is a modification of Equation (35) accounting for the additional contribution of hypnozoite activation to the blood-stage infection burden. However, like the approximation (35) it still arises from replacing a recovery rate that depends on the current value of the MOB with its expectation under steady-state conditions, in which case the MOB would have a Poisson distribution with parameter \((\lambda + i \alpha )/\gamma \).
If a single hypnozoite were established through each infective bite, then the distribution of MOB conditional on the size of the hypnozoite reservoir would be Poisson; for a network of infinite server queues with single arrivals, the numbers of busy servers in each queue follow independent Poisson distributions at each time t (William and Massey andWard Whitt, 1993). The batch structure in hypnozoites established through each infective bite, however, breaks this construction; indeed, Boucherie and Taylor (1993) show that the only open networks of queues that have transient product form distributions are networks of \(M_t/M/\infty \) queues.
The distribution of MOB, conditional on the size of the hypnozoite reservoir, can be recovered using the within-host queueing model introduced in Mehra et al. (2022) (see Sect. 2 for a summary). Suppose the time evolution of the FORI \(\lambda (t)\) is known. Recall that \(N_H(t)\) represents the size of the hypnozoite reservoir at time t and let \(N_B(t) = N_A(t) + N_P(t)\) denote the MOB at time t. By Xekalaki (1987), it follows that
where H denotes the generating function given by Eq. (22). Following similar reasoning to Naasell (2013), given a hypnozoite reservoir of size i, the rate of recovery from (super)infection can be written
Explicit formulae for the rate of recovery from superinfection, in terms of partial Bell polynomials, can be recovered using Leibniz’s integral rule and Faa di Bruno’s formula (Xu and Cen 2011); the resultant expressions, however, are unwieldy and are therefore omitted here. A simpler framework—formulated in terms of a recovery rate corrected for superinfection—is detailed in Appendix D.
D A correction to the model of Anwar et al. (2022)
Anwar et al. (2022) construct a multiscale transmission model for P. vivax, allowing for explicit variation in the hypnozoite burden. The objective of Anwar et al. (2022) is to capture the model structure proposed by White et al. (2014), whilst alleviating the computational difficulties associated with infinite-dimensional compartmental models. The premise of Anwar et al. (2022) is to embed the probabilistic distributions derived on the within-host scale in Mehra et al. (2022) in a transmission model with finitely-many compartments, with the human population stratified by the presence or absence of liver- and/or blood-stage infection.
In particular, as a function of the FORI \(\lambda (t)\), Anwar et al. (2022) derive a system of IDEs governing the time evolution of the prevalence of blood-stage infection \(I_H(t)\) in a homogeneous population. To do so, Anwar et al. (2022) draw on the relapse rate, conditional on the absence of blood-stage infection, that we derive at the within-host level in Mehra et al. (2022).
This within-host framework is designed to capture the joint dynamics of superinfection and the hypnozoite reservoir. As a function of the FORI \(\lambda (\tau )\) in the interval \(\tau \in [0, t)\), using Equation (77) of Mehra et al. (2022), the relapse rate r(t) conditional on the absence of blood-stage infection at time t can be written
While this relapse rate is derived under a framework that takes superinfection into account, the population-level framework proposed in Anwar et al. (2022) does not track the proportion of individuals in the population that have specific values of the MOB, that is it does not account for superinfection in modelling the recovery rate of individuals. In fact, all blood-stage infected individuals are assumed to recover from infection at a constant rate \(\gamma \). The inclusion of superinfection in the work of Anwar et al. (2022) would necessitate the derivation of the recovery rate from (super)infection under the within-host framework of Mehra et al. (2022). As in Naasell (2013), the rate of recovery from (super)infection could be written in terms of the within-host PMF for the MOB \(N_A(t) + N_P(t)\) at time t (given by Equations (81) and (82) of Mehra et al. (2022)):
which, in turn, is a function of the FORI \(\lambda (\tau )\) in the interval \(\tau \in [0, t)\). A rigorous characterisation of superinfection would yield the ODE
rather than
which appeared in Anwar et al. (2022).
However, rather than formulating a model in terms of the relapse rate and the rate of recovery from (super)infection, a simpler but equivalent approach is to directly consider the (integral) expression for the expected prevalence of blood-stage infection in the human population over time under the within-host framework of Mehra et al. (2022), as we have done in this paper.
While the proposed correction to the framework of Anwar et al. (2022) is somewhat subtle, it has important implications. As discussed in Anwar et al. (2022), the omission of superinfection at the population-level leads to discrepancies relative to the framework of White et al. (2014), the re-formulation of which was the primary aim; these discrepancies are evident at high transmission intensities, where superinfections would be expected. The deterministic model introduced in Sect. 4, in contrast, serves as an extension of the framework of White et al. (2014) to allow for superinfection. The omission of superinfection at the population-level in Anwar et al. (2022) means that a number of key epidemiological parameters that are analytically tractable at the within-host level do not make sense at the population-level. A key example is the expected contribution of relapse vs primary infection to the overall burden of recurrence (which includes the case of overlapping relapses and primary infections, as in Mehra et al. (2022)).
E The model of Bailey (1957)
In the case \(\nu =0\) (that is, the absence of hypnozoite accrual), Eqs. (20) and (21) reduce to the classical (deterministic) model of superinfection for P. falciparum formulated by Bailey (1957).
In the case \(\nu =0\), Eq. (20) yields the Kolmogorov forward differential equations for the \(M_t/M/\infty \) queue. By Eick et al. (1993), it follows that the MOB in the human population at time t necessarily has a Poisson distribution. The mean MOB
can be written a function of the FORI \(aI_M(\tau )\) in the interval [0, t), with the final equality following from Eq. (24).
In this case, we can collapse the infinite-dimensional system of ODEs given by Eqs. (20) and (21) into the two-dimensional system
by differentiating Eq. (24). When the MOB in the human population is initially Poisson-distributed, the time evolution of FORI is equivalent under the two-dimensional system of ODEs given by Eqs. (38) and (39), and the countable system of ODEs proposed by Bailey (1957) (Eqs. (20) and (21) in the case \(\nu =0\)).
Noting the functional dependence between the mean MOB \(m_b(t)\) and the prevalence of blood-stage infection \(I_H(t)\) at time t, we observe that the system of ODEs given by Eqs. (38) and (39) is equivalent to the “hybrid approximation” proposed by Nåsell (1991), whereby host and vector dynamics are coupled through expected values.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mehra, S., McCaw, J.M. & Taylor, P.G. Superinfection and the hypnozoite reservoir for Plasmodium vivax: a general framework. J. Math. Biol. 88, 7 (2024). https://doi.org/10.1007/s00285-023-02014-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-023-02014-3