Abstract
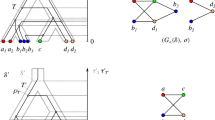
Horizontal gene transfer (HGT) is an important factor for the evolution of prokaryotes as well as eukaryotes. According to Walter M. Fitch, two genes are xenologs if they are separated by at least one HGT. This concept is formalized through Fitch relations, which are defined as binary relations that comprise all pairs (x, y) of genes x and y for which y has been horizontally transferred at least once since it diverged from the last common ancestor of x and y. This definition, in particular, preserves the directional character of the transfer. Fitch relations are characterized by a small set of forbidden induced subgraphs on three vertices and can be recognized in linear time. The mathematical characterization of Fitch relations is crucial to understand whether putative xenology relations are at least to some extent “biologically feasible”. In this contribution, we provide two novel characterizations of Fitch relations. In particular, these results allow us directly to reconstruct gene trees (together with the location of the horizontal transfer events) that explain the underlying Fitch relation. As a biological side result, we can conclude that the phylogenetic signal to infer these gene trees is entirely contained in those pairs of genes x and y for which no directional transfer has been taken place in the common history of y and the last common ancestor of x and y. In other words, non-HGT events provide the essential information about the gene trees. In addition, we utilize the new characterizations to present an alternative, short and elegant proof of the characterization theorem established by Geiß et al. (J Math Bio 77(5), 2018).



Similar content being viewed by others
References
Abascal F, Posada D, Zardoya R (2012) The evolution of the mitochondrial genetic code in arthropods revisited. Mitochondrial DNA 23:84–91
Altenhoff AM, Glover NM, Train CM, Kaleb K, Warwick Vesztrocy A, Dylus D, de Farias TM, Zile K, Stevenson C, Long J et al (2017) The OMA orthology database in 2018: retrieving evolutionary relationships among all domains of life through richer web and programmatic interfaces. Nucleic Acids Res 46:D477–D485
Barlow M (2009) What antimicrobial resistance has taught us about horizontal gene transfer. Humana Press, Totowa, pp 397–411
Böcker S, Dress AWM (1998) Recovering symbolically dated, rooted trees from symbolic ultrametrics. Adv Math 138:105–125
Boore JL (2006) The use of genome-level characters for phylogenetic reconstruction. Trends Ecol Evol 21:439–446
Boore JL, Brown WM (1998) Big trees from little genomes: mitochondrial gene order as a phylogenetic tool. Curr Opin Genet Dev 8:668–674
Corneil DG, Lerchs H, Steward Burlingham L (1981) Complement reducible graphs. Discr Appl Math 3:163–174
Delsuc F, Brinkmann H, Philippe H (2005) Phylogenomics and the reconstruction of the tree of life. Nat Rev Genet 6:361–375
Donath A, Stadler PF (2014) Molecular morphology: Higher order characters derivable from sequence information. In: Wägele JW, Bartolomaeus T (eds) Deep metazoan phylogeny: the backbone of the tree of life. New insights from analyses of molecules, morphology, and theory of data analysis. de Gruyter, Berlin, pp 549–562
Dutilh BE, Snel B, Ettema TJ, Huynen MA (2008) Signature genes as a phylogenomic tool. Mol Biol Evol 25:1659–1667
Fitch WM (1970) Distinguishing homologous from analogous proteins. Syst Biol 19:99–113
Fitch WM (2000) Homology a personal view on some of the problems. Trends Genet 16:227–231
Gabaldón T, Koonin E (2013) Functional and evolutionary implications of gene orthology. Nat Rev Genet 14:360–366. https://doi.org/10.1038/nrg3456
Geiß M, Anders J, Stadler PF, Wieseke N, Hellmuth M (2018) Reconstructing gene trees from Fitch’s xenology relation. J Math Biol 77:1459–1491
Gogarten JP, Townsend JP (2005) Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol 3:679
Goodman M, Czelusniak J, Moore GW, Romero-Herrera AE, Matsuda G (1979) Fitting the gene lineage into its species lineage, a parsimony strategy illustrated by cladograms constructed from globin sequences. Syst Biol 28:132–163
Hellmuth M, Geiß M, Long Y, Stadler P (2018a) A short note on undirected Fitch graphs. Art Discrete Appl Math 1(P1):08
Hellmuth M, Hernandez-Rosales M, Huber KT, Moulton V, Stadler PF, Wieseke N (2013) Orthology relations, symbolic ultrametrics, and cographs. J Math Biol 66:399–420
Hellmuth M, Hernandez-Rosales M, Long Y, Stadler P (2018b) Inferring phylogenetic trees from the knowledge of rare evolutionary events. J Math Biol 76:1623–1653
Hellmuth M, Stadler PF, Wieseke N (2017) The mathematics of xenology: di-cographs, symbolic ultrametrics, 2-structures and tree-representable systems of binary relations. J Math Biol 75:199–237
Hellmuth M, Wieseke N (2016) From sequence data including orthologs, paralogs, and xenologs to gene and species trees. In: Pontarotti P (ed) Evolutionary biology: convergent evolution, evolution of complex traits, concepts and methods. Springer, Cham, pp 373–392
Hellmuth M, Wieseke N, Lechner M, Lenhof HP, Middendorf M, Stadler PF (2015) Phylogenomics with paralogs. Proc Natl Acad Sci 112:2058–2063
Hirt RP, Alsmark C, Embley TM (2015) Lateral gene transfers and the origins of the eukaryote proteome: a view from microbial parasites. Curr Opin Microbiol 23:155–162
Hotopp JCD (2011) Horizontal gene transfer between bacteria and animals. Trends Genet 27:157–163
Jain R, Rivera MC, Moore JE, Lake JA (2003) Horizontal gene transfer accelerates genome innovation and evolution. Mol Biol Evol 20:1598–1602
Jensen RA (2001) Orthologs and paralogs—we need to get it right. Genome Biol 2:interactions1002
Keeling PJ, Palmer JD (2008) Horizontal gene transfer in eukaryotic evolution. Nat Rev Genet 9:605
Koonin EV (2005) Orthologs, paralogs, and evolutionary genomics. Annu Rev Genet 39:309–338
Lavrov DV (2007) Key transitions in animal evolution: a mitochondrial DNA perspective. Integr Comp Biol 47:734–743
Lawrence JG, Ochman H (2002) Reconciling the many faces of lateral gene transfer. Trends Microbiol 10:1–4
Lechner M, Findeiß S, Steiner L, Marz M, Stadler PF, Prohaska SJ (2011) Proteinortho: detection of (co-)orthologs in large-scale analysis. BMC Bioinformatics 12:124
Lechner M, Hernandez-Rosales M, Doerr D, Wiesecke N, Thevenin A, Stoye J, Hartmann RK, Prohaska SJ, Stadler PF (2014) Orthology detection combining clustering and synteny for very large datasets. PLoS ONE 9:e105015. https://doi.org/10.1371/2Fjournal.pone.0105015
Mahmood K, Webb GI, Song J, Whisstock JC, Konagurthu AS (2012) Efficient large-scale protein sequence comparison and gene matching to identify orthologs and co-orthologs. Nucleic Acids Res 40:e44–e44
Mushegian AR, Koonin EV (1996) A minimal gene set for cellular life derived by comparison of complete bacterial genomes. Proc Natl Acad Sci USA 93:10268–10273
Ochman H, Lawrence JG, Groisman EA (2000) Lateral gene transfer and the nature of bacterial innovation. Nature 405:299
Rancurel C, Legrand L, Danchin EGJ (2017) Alienness: rapid detection of candidate horizontal gene transfers across the tree of life. Genes 8:E248
Ravenhall M, Škunca N, Lassalle F, Dessimoz C (2015) Inferring horizontal gene transfer. PLoS Comput Biol 11:e1004095
Rogozin IB, Sverdlov AV, Babenko VN, Koonin EV (2005) Analysis of evolution of exon-intron structure of eukaryotic genes. Brief Bioinform 6:118–134
Rokas A, Holland PW (2000) Rare genomic changes as a tool for phylogenetics. Trends Ecol Evol 15:454–459
Sankoff D, Leduc G, Antoine N, Paquin B, Lang BF, Cedergren R (1992) Gene order comparisons for phylogenetic inference: evolution of the mitochondrial genome. Proc Natl Acad Sci USA 89:6575–6579
Savory F, Leonard G, Richards TA (2015) The role of horizontal gene transfer in the evolution of the oomycetes. PLoS Pathog 11:e1004805
Sempere LF, Cole CN, McPeek MA, Peterson KJ (2006) The phylogenetic distribution of metazoan microRNAs: insights into evolutionary complexity and constraint. J Exp Zoolog B Mol Dev Evol 306:575–588
Semple C, Steel M (2003) Phylogenetics, vol. 24 of Oxford Lecture Series in Mathematics and its Applications. Oxford University Press, Oxford
Sonnhammer E, Östlund G (2015) Inparanoid 8: orthology analysis between 273 proteomes, mostly eukaryotic. Nucleic Acids Res 43:D234–D239
Steel M (2016) Phylogeny: discrete and random processes in evolution. In: CBMS-NSF regional conference series in applied mathematics. Society for Industrial and Applied Mathematics, Philadelphia
Syvanen M, Kado CI (2001) Horizontal gene transfer. Academic, Cambridge
Tatusov RL, Galperin MY, Natale DA, Koonin EV (2000) The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res 28:33–36
Tatusov RL, Koonin EV, Lipman DJ (1997) A genomic perspective on protein families. Science 278:631–637
Wapinski I, Pfeffer A, Friedman N, Regev A (2007) Automatic genome-wide reconstruction of phylogenetic gene trees. Bioinformatics 23:i549–i558
Acknowledgements
We are grateful to Manuela Geiß and Peter F. Stadler for all the fruitful and interesting discussions. Moreover, we thank Carmen Bruckmann and Annemarie Luise Kühn for their constructive comments and suggestions that helped to improve the paper. The authors would also like to thank the anonymous referees for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hellmuth, M., Seemann, C.R. Alternative characterizations of Fitch’s xenology relation. J. Math. Biol. 79, 969–986 (2019). https://doi.org/10.1007/s00285-019-01384-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-019-01384-x
Keywords
- Fitch xenology
- Fitch relation
- Phylogenetic tree
- Horizontal gene transfer
- Forbidden induced subgraphs
- Neighborhoods
- Gene evolution