Abstract
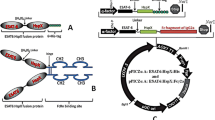
An urgent need is to introduce an effective vaccine against Mycobacterium tuberculosis (M.tb) infection. In the present study, a multi-stage M.tb immunodominant Fcγ1 fusion protein (Ag85B:HspX:hFcγ1) was designed and produced, and the immunogenicity of purified protein was evaluated. This recombinant fusion protein was produced in the Pichia pastoris expression system. The HiTrap-rPA column affinity chromatography purified and confirmed the fusion protein using ELISA and Western blotting methods. The co-localisation assay was used to confirm its proper folding and function. IFN-γ, IL-12, IL-4, and TGF-β expression in C57BL/6 mice then evaluated the immunogenicity of the construct in the presence and absence of BCG. After expression optimisation, medium-scale production and the Western blotting test confirmed suitable production of Ag85B:HspX:hFcγ1. The co-localisation results on antigen-presenting cells (APCs) showed that Ag85B:HspX:hFcγ1 properly folded and bound to hFcγRI. This strong co-localisation with its receptor can confirm inducing proper Th1 responses. The in vivo immunisation assay showed no difference in the expression of IL-4 but a substantial increase in the expression of IFN-γ and IL-12 (P ≤ 0.02) and a moderate increase in TGF-β (P = 0.05). In vivo immunisation assay revealed that Th1-inducing pathways have been stimulated, as IFN-γ and IL-12 strongly, and TGF-β expression moderately increased in Ag85B:HspX:hFcγ1 group and Ag85B:HspX:hFcγ1+BCG. Furthermore, the production of IFN-γ from splenocytes in the Ag85B:HspX:hFcγ1 group was enormously higher than in other treatments. Therefore, this Fc fusion protein can make a selective multi-stage delivery system for inducing appropriate Th1 responses and is used as a subunit vaccine alone or in combination with others.




Similar content being viewed by others
Data Availability
All data supporting this study's findings are included in the manuscript and available from the corresponding author upon reasonable request.
References
Egedesø PJ, Hansen CW, Jensen PS (2020) Preventing the white death: tuberculosis dispensaries. Econ J 130(629):1288–1316. https://doi.org/10.1093/ej/ueaa014
Cliff JM, Kaufmann SH, McShane H, van Helden P, O’Garra A (2015) The human immune response to tuberculosis and its treatment: a view from the blood. Immunol Rev 264(1):88–102. https://doi.org/10.1111/imr.12269
Purmohamad A, Azimi T, Nasiri MJ, Goudarzi M, Zangiabadian M, Sedighian H et al (2020) HIV-tuberculous meningitis co-infection: a systematic review and meta-analysis. Curr Pharm Biotechnol. https://doi.org/10.2174/1389201021666200730143906
Mahajan NS, Dhawale S (2015) Linked pyridinyl-thiadiazoles: design and synthesis as potential candidate for treatment of XDR and MDR tuberculosis. Eur J Med Chem 102:243–248
Fatima S, Kumari A, Das G, Dwivedi VP (2020) Tuberculosis vaccine: a journey from BCG to present. Life Sci 252:117594. https://doi.org/10.1016/j.lfs.2020.117594
Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM et al (2013) Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 58(4):470–480. https://doi.org/10.1093/cid/cit790
Khoshnood S, Heidary M, Haeili M, Drancourt M, Darban-Sarokhalil D, Nasiri MJ et al (2018) Novel vaccine candidates against Mycobacterium tuberculosis. Int J Biol Macromol 120(Pt A):180–188. https://doi.org/10.1016/j.ijbiomac.2018.08.037
Kaufmann SH, Weiner J, von Reyn CF (2017) Novel approaches to tuberculosis vaccine development. Int J Infect Dis 56:263–267. https://doi.org/10.1016/j.ijid.2016.10.018
Aagaard C, Hoang T, Dietrich J, Cardona P-J, Izzo A, Dolganov G et al (2011) A multi-stage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 17(2):189
Farsiani H, Mosavat A, Soleimanpour S, Sadeghian H, Akbari Eydgahi MR, Ghazvini K et al (2016) Fc-based delivery system enhances immunogenicity of a tuberculosis subunit vaccine candidate consisting of the ESAT-6:CFP-10 complex. Mol Biosyst 12(7):2189–2201. https://doi.org/10.1039/c6mb00174b
Kebriaei A, Derakhshan M, Meshkat Z, Eidgahi MR, Rezaee SA, Farsiani H et al (2016) Construction and immunogenicity of a new Fc-based subunit vaccine candidate against Mycobacterium tuberculosis. Mol Biol Rep 43(9):911–922. https://doi.org/10.1007/s11033-016-4024-9
Mosavat A, Soleimanpour S, Farsiani H, Sadeghian H, Ghazvini K, Sankian M et al (2016) Fused Mycobacterium tuberculosis multi-stage immunogens with an Fc-delivery system as a promising approach for the development of a tuberculosis vaccine. Infect Genet Evol 39:163–172
Babaki MKZ, Taghiabadi M, Soleimanpour S, Moghadam MS, Mosavat A, Amini AA et al (2019) Mycobacterium tuberculosis Ag85b: hfcγ1 recombinant fusion protein as a selective receptor-dependent delivery system for antigen presentation. Microb Pathog 129:68–73
Abebe F (2019) Synergy between Th1 and Th2 responses during Mycobacterium tuberculosis infection: a review of current understanding. Int Rev Immunol 38(4):172–179. https://doi.org/10.1080/08830185.2019.1632842
Amelio P, Portevin D, Reither K, Mhimbira F, Mpina M, Tumbo A et al (2017) Mixed Th1 and Th2 Mycobacterium tuberculosis-specific CD4 T cell responses in patients with active pulmonary tuberculosis from Tanzania. PLoS Negl Trop Dis 11(7):e0005817. https://doi.org/10.1371/journal.pntd.0005817
Cardona P, Cardona PJ (2019) Regulatory T cells in Mycobacterium tuberculosis infection. Front Immunol 10:2139. https://doi.org/10.3389/fimmu.2019.02139
Babaki MKZ, Soleimanpour S, Rezaee SA (2017) Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene: biology, immune-pathogenicity, applications in diagnosis, and vaccine design. Microb Pathog 112:20–29
Wiker HG, Harboe M (1992) The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev 56(4):648–661
Forrellad MA, Klepp LI, Gioffré A, Sabio y Garcia J, Morbidoni HR, Santangelo MDLP, Cataldi AA, Bigi F (2013) Virulence factors of the Mycobacterium tuberculosis complex. Virulence 4(1):3–66. https://doi.org/10.4161/viru.22329
Kuo CJ, Bell H, Hsieh CL, Ptak CP, Chang YF (2012) Novel mycobacteria antigen 85 complex binding motif on fibronectin. J Biol Chem 287(3):1892–1902. https://doi.org/10.1074/jbc.M111.298687
Ramachandra L, Smialek JL, Shank SS, Convery M, Boom WH, Harding CV (2005) Phagosomal processing of Mycobacterium tuberculosis antigen 85B is modulated independently of mycobacterial viability and phagosome maturation. Infect Immun 73(2):1097–1105. https://doi.org/10.1128/iai.73.2.1097-1105.2005
Huygen K (2014) The immunodominant T-cell epitopes of the mycolyl-transferases of the antigen 85 complex of M. tuberculosis. Front Immunol 5:321. https://doi.org/10.3389/fimmu.2014.00321
Dey B, Jain R, Gupta UD, Katoch VM, Ramanathan VD, Tyagi AK (2011) A booster vaccine expressing a latency-associated antigen augments BCG induced immunity and confers enhanced protection against tuberculosis. PLoS One 6(8):e23360. https://doi.org/10.1371/journal.pone.0023360
Taylor JL, Wieczorek A, Keyser AR, Grover A, Flinkstrom R, Karls RK et al (2012) HspX-mediated protection against tuberculosis depends on its chaperoning of a mycobacterial molecule. Immunol Cell Biol 90(10):945–954
Levin D, Golding B, Strome SE, Sauna ZE (2015) Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol 33(1):27–34
Mohammadzadeh R, Karbalaei M, Soleimanpour S, Mosavat A, Rezaee SA, Ghazvini K et al (2021) Practical methods for expression of recombinant protein in the Pichia pastoris system. Curr Protoc 1(6):1155. https://doi.org/10.1002/cpz1.155
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882. https://doi.org/10.1093/nar/25.24.4876
Krieger E, Joo K, Lee J, Lee J, Raman S, Thompson J et al (2009) Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: four approaches that performed well in CASP8. Proteins 77(9):114–122
Krieger E, Koraimann G, Vriend G (2002) Increasing the precision of comparative models with YASARA NOVA—a self-parameterising force field. Proteins 47(3):393–402
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2(9):1511–1519. https://doi.org/10.1002/pro.5560020916
Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrøm T, Agger EM, Andersen P et al (2010) Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J Control Release 145(2):102–108. https://doi.org/10.1016/j.jconrel.2010.03.027
Tarokhian H, Rahimi H, Mosavat A, Shirdel A, Rafatpanah H, Akbarin MM et al (2018) HTLV-1-host interactions on the development of adult T cell leukemia/lymphoma: virus and host gene expressions. BMC Cancer 18(1):1287. https://doi.org/10.1186/s12885-018-5209-5
Mao F, Leung W-Y, Xin X (2007) Characterization of EvaGreen and the implication of its physicochemical properties for qPCR applications. BMC Biotechnol 7(1):76. https://doi.org/10.1186/1472-6750-7-76
Ramezani S, Shirdel A, Rafatpanah H, Akbarin MM, Tarokhian H, Rahimi H et al (2017) Assessment of HTLV-1 proviral load, LAT, BIM, c-FOS and RAD51 gene expression in adult T cell leukemia/lymphoma. Med Microbiol Immunol 206(4):327–335. https://doi.org/10.1007/s00430-017-0506-1
Zeinali M, Shafaei A, Rafatpanah H, Mosavat A, Tayebi-Meybodi N, Hosseinzadeh H et al (2022) Potential protective effects of chrysin against immunotoxicity induced by diazinon. Sci Rep 12(1):15578. https://doi.org/10.1038/s41598-022-20010-3
Wang Z, Wang Y, Zhang D, Li J, Hua Z, Du G et al (2010) Enhancement of cell viability and alkaline polygalacturonate lyase production by sorbitol co-feeding with methanol in Pichia pastoris fermentation. Bioresour Technol 101(4):1318–1323. https://doi.org/10.1016/j.biortech.2009.09.025
Adamova E, Walsh MC, Gosselin DR, Hale K, Preissler MT, Graziano RF et al (2005) Enhanced antigen-specific antibody and cytokine responses when targeting antigen to human FcGAMMA receptor type I using an anti-human FcGAMMA receptor type I-streptavidin fusion protein in an adjuvant-free system. Immunol Invest 34(4):417–429
Keler T, Guyre PM, Vitale LA, Sundarapandiyan K, van De Winkel JG, Deo YM et al (2000) Targeting weak antigens to CD64 elicits potent humoral responses in human CD64 transgenic mice. J Immunol 165(12):6738–6742. https://doi.org/10.4049/jimmunol.165.12.6738
Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV et al (2008) Utilisation of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol 180(8):5548–5557
Czajkowsky DM, Hu J, Shao Z, Pleass RJ (2012) Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med 4(10):1015–1028
Agger EM, Rosenkrands I, Hansen J, Brahimi K, Vandahl BS, Aagaard C et al (2008) Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One 3(9):e3116. https://doi.org/10.1371/journal.pone.0003116
Tang XL, Zhou YX, Wu SM, Pan Q, Xia B, Zhang XL (2014) CFP10 and ESAT6 aptamers as effective Mycobacterial antigen diagnostic reagents. J Infect 69(6):569–580. https://doi.org/10.1016/j.jinf.2014.05.015
Sharebiani H, Hajimiri S, Abbasnia S, Soleimanpour S, Hashem Asnaashari AM, Valizadeh N et al (2021) Game theory applications in host-microbe interactions toward disease manifestation: Mycobacterium tuberculosis infection as an example. Iran J Basic Med Sci 24(10):1324–1335
Soleimanpour S, Farsiani H, Mosavat A, Ghazvini K, Eydgahi MR, Sankian M et al (2015) APC targeting enhances immunogenicity of a novel multi-stage Fc-fusion tuberculosis vaccine in mice. Appl Microbiol Biotechnol 99(24):10467–10480. https://doi.org/10.1007/s00253-015-6952-z
Bhatt K, Salgame P (2007) Host innate immune response to Mycobacterium tuberculosis. J Clin Immunol 27(4):347–362. https://doi.org/10.1007/s10875-007-9084-0
Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM et al (2001) Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 292(5523):1907–1910
Pitt JM, Blankley S, McShane H, O’Garra A (2013) Vaccination against tuberculosis: how can we better BCG? Microb Pathog 58:2–16
Lew MH, Norazmi MN, Tye GJ (2020) Enhancement of immune response against Mycobacterium tuberculosis HspX antigen by incorporation of combined molecular adjuvant (CASAC). Mol Immunol 117:54–64. https://doi.org/10.1016/j.molimm.2019.10.023
Junker F, Gordon J, Qureshi O (2020) Fc gamma receptors and their role in antigen uptake, presentation, and T cell activation. Front Immunol 11:1393. https://doi.org/10.3389/fimmu.2020.01393
Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M et al (2007) T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity 27(3):505–517. https://doi.org/10.1016/j.immuni.2007.07.022
Reljic R, Paul MJ, Arias MA (2009) Cytokine therapy of tuberculosis at the crossroads. Expert Rev Respir Med 3(1):53–66. https://doi.org/10.1586/17476348.3.1.53
Soleimanpour S, Hassannia T, Motiee M, Amini AA, Rezaee SA (2017) Fcγ1 fragment of IgG1 as a powerful affinity tag in recombinant Fc-fusion proteins: immunological, biochemical and therapeutic properties. Crit Rev Biotechnol 37(3):371–392. https://doi.org/10.3109/07388551.2016.1163323
Konduru K, Bradfute SB, Jacques J, Manangeeswaran M, Nakamura S, Morshed S et al (2011) Ebola virus glycoprotein Fc fusion protein confers protection against lethal challenge in vaccinated mice. Vaccine 29(16):2968–2977. https://doi.org/10.1016/j.vaccine.2011.01.113
Loureiro S, Ren J, Phapugrangkul P, Colaco CA, Bailey CR, Shelton H et al (2011) Adjuvant-free immunisation with hemagglutinin-Fc fusion proteins as an approach to influenza vaccines. J Virol 85(6):3010–3014. https://doi.org/10.1128/JVI.01241-10
Ren W, Sun H, Gao GF, Chen J, Sun S, Zhao R et al (2020) Recombinant SARS-CoV-2 spike S1-Fc fusion protein induced high levels of neutralising responses in nonhuman primates. Vaccine 38(35):5653–5658. https://doi.org/10.1016/j.vaccine.2020.06.066
Acknowledgements
The authors thank the National Institute for Medical Research Development (NIMAD), Tehran, Iran, for granting this study [NIMAD 958012, recipient: SAR. Rezaee]. The authors especially thank the Vice-Chancellor for Research and Technology, Mashhad University of Medical Sciences (MUMS), Mashhad, Iran.
The results described in this paper were part of the student thesis. This study was subjected to a Ph.D. thesis in Medical Bacteriology by Mohsen Karbalaei at MUMS, under Grant [MUMS 951414, recipient: SAR. Rezaee]. The experiments were conducted in Immunology Research Lab, Inflammation and Inflammatory Diseases Division, Immunology Research Center, and Antimicrobial Resistance Research Center, the Mashhad University of Medical Sciences, Mashhad, Iran.
Funding
This study was financially supported by the National Institute for Medical Research Development, Tehran, Iran, under Grant [NIMAD 958012, recipient: SAR. Rezaee] and the Vice-Chancellor for Research and Technology, Mashhad University of Medical Sciences, Mashhad, Iran, under Grant [MUMS 951414, recipient: SAR. Rezaee].
Author information
Authors and Affiliations
Contributions
Doing experiments and manuscript drafting: MK, AM, SS, and HF; In Silico protein modelling: AA; Research advisors: SS, HF, MS, and AM; Research director, Conception and design of the study, and Data analysis: SAR and KG. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
This study was the subject of a Patent for the National Institute for Medical Research Development in the territory of Iran (NIMAD). The authors declare no conflict of interest regarding this manuscript in other regions.
Ethical Approval
The animal experiments were reviewed and approved by the Biomedical Research Ethics Committee of NIMAD [IR.NIMAD.REC.958012] that was performed according to National Institutes of Health guidelines (NIH publication No. 85-23, revised 1985). All methods were performed following relevant guidelines and regulations. All surgery was performed under sodium pentobarbital anaesthesia, and all efforts were made to minimise suffering. During the experiments, the vaccinated mice were monitored every day.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karbalaei, M., Mosavat, A., Soleimanpour, S. et al. Production and Evaluation of Ag85B:HspX:hFcγ1 Immunogenicity as an Fc Fusion Recombinant Multi-Stage Vaccine Candidate Against Mycobacterium tuberculosis. Curr Microbiol 81, 127 (2024). https://doi.org/10.1007/s00284-024-03655-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03655-3