Abstract
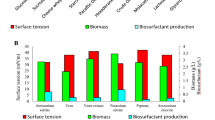
Lignocellulosic material is one of the raw materials that can be used to reduce the cost of biosurfactant production because it is cheap, abundantly available, and contains cellulose and hemicellulose which can be hydrolyzed to glucose and xylose as carbon sources. This study aimed to evaluate biosurfactant production by Bacillus species using glucose and xylose as carbon sources, which are the most abundant sugar monomers from the hydrolysis of lignocellulosic materials. In this study, biosurfactants were produced by six bacterial isolates belonging to the Bacillus genus. The six bacterial isolates were identified molecularly through 16S rRNA sequencing. The results showed that the six bacterial isolates were identified as B. subtilis ITBCC46, B. subtilis ITBCC40, B. subtilis ITBCC31, B. siamensis ITBCC36, B. xiamenensis ITBCC43, and B. subtilis ITBCC30. All Bacillus species used in this study could be grown on glucose or xylose media. Biosurfactants produced by B. subtilis ITBCC46, B. subtilis ITBCC40, B. subtilis ITBCC31, and B. siamensis ITBCC36 could reduce surface tension below 40 mN/m (32.70 to 39.15 mN/m). All biosurfactants produced by these Bacillus species had more than 50% emulsification stability. These characteristics indicated that the biosurfactants had the desired quality.




Similar content being viewed by others
Data Availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request. All datasets analyzed during this study are included in this published article.
References
Sakthipriya N, Doble M, Sangwai JS (2015) Fast degradation and viscosity reduction of waxy crude oil and model waxy crude oil using Bacillus subtilis. J Petrol Sci Eng 134:158–166. https://doi.org/10.1016/j.petrol.2015.08.002
Geetha SJ, Banat IM, Joshi SJ (2018) Biosurfactants: Production and potential applications in microbial enhanced oil recovery (MEOR). Biocatal Agric Biotechnol 14:23–32. https://doi.org/10.1016/j.bcab.2018.01.010
Kiran GS, Thajuddin N, Hema TA et al (2010) Optimization and characterization of rhamnolipid biosurfactant from sponge associated marine fungi Aspergillus sp. MSF1. Desalin Water Treat 24:257–265. https://doi.org/10.5004/dwt.2010.1569
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Renga Thavasi T, Marchant R, Banat I (2014) Biosurfactant Applications in Agriculture. In: Kosaric N, Sukan FV (eds) Biosurfactants: Production and Utilization-Processes, Technologies, and Economics, Editors: Kosaric and Sukan, CRC Press, Boca Raton, pp 313–326. https://doi.org/10.1201/b17599-18
Santos DKF, Meira HM, Rufino RD et al (2017) Biosurfactant production from Candida lipolytica in bioreactor and evaluation of its toxicity for application as a bioremediation agent. Process Biochem 54:20–27. https://doi.org/10.1016/j.procbio.2016.12.020
Rane AN, Baikar VV, Ravi Kumar V, Deopurkar RL (2017) Agro-industrial wastes for production of biosurfactant by Bacillus subtilis ANR 88 and its application in synthesis of silver and gold nanoparticles. Front Microbiol 8:492–492. https://doi.org/10.3389/fmicb.2017.00492
Nitschke M, Costa SGVAO, Haddad R et al (2005) Oil wastes as unconventional substrates for rhamnolipid biosurfactant production by Pseudomonas aeruginosa LBI. Biotechnol Prog 21:1562–1566. https://doi.org/10.1021/bp050198x
Wadekar S, Kale S, Lali A et al (2012) Utilization of sweetwater as a cost-effective carbon source for sophorolipids production by Starmerella bombicola (ATCC 22214). Prep Biochem Biotechnol 42:125–142. https://doi.org/10.1080/10826068.2011.577883
Campos JM, Stamford TLM, Sarubbo LA et al (2013) Microbial biosurfactants as additives for food industries. Biotechnol Prog 29:1097–1108. https://doi.org/10.1002/btpr.1796
Faria NT, Santos MV, Fernandes P et al (2014) Production of glycolipid biosurfactants, mannosylerythritol lipids, from pentoses and d-glucose/d-xylose mixtures by Pseudozyma yeast strains. Process Biochem 49:1790–1799. https://doi.org/10.1016/j.procbio.2014.08.004
Chaturvedi V, Verma P (2013) An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech. https://doi.org/10.1007/s13205-013-0167-8
Gírio FM, Fonseca C, Carvalheiro F et al (2010) Hemicelluloses for fuel ethanol: a review. Biores Technol 101:4775–4800. https://doi.org/10.1016/j.biortech.2010.01.088
Liu Y, Rainey PB, Zhang X-X (2015) Molecular mechanisms of xylose utilization by Pseudomonas fluorescens: overlapping genetic responses to xylose, xylulose, ribose and mannitol. Mol Microbiol 98:553–570. https://doi.org/10.1111/mmi.13142
Jeffries TW (1983) Utilization of xylose by bacteria, yeasts, and fungi. Pentoses and Lignin. Springer, Heidelberg, pp 1–32
Hameş EE, Vardar-Sukan F, Kosaric N (2014) Patents on biosurfactants and future trends. biosurfactants: production and utilization-processes. Technol Econ 159:165–244
Nogueira Felix AK, Martins JJL, Lima Almeida JG et al (2019) Purification and characterization of a biosurfactant produced by Bacillus subtilis in cashew apple juice and its application in the remediation of oil-contaminated soil. Colloids Surf, B 175:256–263. https://doi.org/10.1016/j.colsurfb.2018.11.062
Chen C, Lin J, Wang W et al (2019) Cost-effective production of surfactin from Xylose-rich corncob hydrolysate using Bacillus subtilis BS-37. Waste Biomass Valor 10:341–347. https://doi.org/10.1007/s12649-017-0052-5
Stancu MM (2020) Biosurfactant production by a Bacillus megaterium strain. Open Life Sciences 15:629–637. https://doi.org/10.1515/biol-2020-0068
Purwasena IA, Astuti DI, Syukron M et al (2019) Stability test of biosurfactant produced by Bacillus licheniformis DS1 using experimental design and its application for MEOR. J Pet Sci Eng 183:106383. https://doi.org/10.1016/j.petrol.2019.106383
Fooladi T, Abdeshahian P, Moazami N et al (2018) Enhanced biosurfactant production by Bacillus pumilus 2IR in fed-batch fermentation using 5-L bioreactor. Iran J Sci Technol, Trans A: Sci 42:1111–1123. https://doi.org/10.1007/s40995-018-0599-4
Chaurasia LK, Tamang B, Tirwa RK, Lepcha PL (2020) Influence of biosurfactant producing Bacillus tequilensis LK5.4 isolate of kinema, a fermented soybean, on seed germination and growth of maize (Zea mays L.). 3 Biotech 10:297. https://doi.org/10.1007/s13205-020-02281-7
Zhang J, Feng W, Xue Q (2022) Biosurfactant production and oil degradation by Bacillus siamensis and its potential applications in enhanced heavy oil recovery. Int Biodeterior Biodegrad 169:105388. https://doi.org/10.1016/j.ibiod.2022.105388
Domingues R, Bondar M, Palolo I et al (2021) Xylose metabolism in bacteria—opportunities and challenges towards efficient lignocellulosic biomass-based biorefineries. Appl Sci 11:8112. https://doi.org/10.3390/app11178112
Miftakhurohmah MKH, Soekarno BPW et al (2021) Identification of endogenous and episomal piper yellow mottle virus from the leaves and berries of black pepper (Piper nigrum). Australas Plant Pathol 50:431–434. https://doi.org/10.1007/s13313-021-00791-3
Pereira JFB, Gudiña EJ, Costa R et al (2013) Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 111:259–268. https://doi.org/10.1016/j.fuel.2013.04.040
Al-Wahaibi Y, Joshi S, Al-Bahry S et al (2014) Biosurfactant production by Bacillus subtilis B30 and its application in enhancing oil recovery. Colloids Surf, B 114:324–333. https://doi.org/10.1016/j.colsurfb.2013.09.022
Gomaa E, El-Mehy R (2019) Bacterial biosurfactant from Citrobacter freundii MG8123141 as a bioremoval tool of heavy metals from wastewater. Bull Natl Res Cent. https://doi.org/10.1186/s42269-019-0088-8
Freitas de Oliveira DW, Lima França ÍW, Nogueira Félix AK et al (2013) Kinetic study of biosurfactant production by Bacillus subtilis LAMI005 grown in clarified cashew apple juice. Colloids Surf, B 101:34–43. https://doi.org/10.1016/j.colsurfb.2012.06.011
Al-Sulaimani H, Al-Wahaibi Y, Al-Bahry S et al (2011) Optimization and partial characterization of biosurfactants produced by Bacillus Species and their potential for Ex-situ enhanced oil recovery. SPE J 16:672–682
Van Rossum T, Ferretti P, Maistrenko OM, Bork P (2020) Diversity within species: interpreting strains in microbiomes. Nat Rev Microbiol 18:491–506. https://doi.org/10.1038/s41579-020-0368-1
Foysal MJ, Lisa AK (2018) Isolation and characterization of Bacillus sp. strain BC01 from soil displaying potent antagonistic activity against plant and fish pathogenic fungi and bacteria. J Genet Eng Biotechnol 16:387–392. https://doi.org/10.1016/j.jgeb.2018.01.005
Prado AAOS, Santos BLP, Vieira IMM et al (2019) Evaluation of a new strategy in the elaboration of culture media to produce surfactin from hemicellulosic corncob liquor. Biotechnol Rep 24:e00364. https://doi.org/10.1016/j.btre.2019.e00364
Nurfarahin AH, Mohamed MS, Phang LY (2018) Culture medium development for microbial-derived surfactants production—an overview. Molecules. https://doi.org/10.3390/molecules23051049
Hsieh F-C, Li M-C, Lin T-C, Kao S-S (2004) Rapid detection and characterization of surfactin-producing Bacillus subtilis and Closely related species based on PCR. Curr Microbiol 49:186–191. https://doi.org/10.1007/s00284-004-4314-7
Winterburn JB, Martin PJ (2012) Foam mitigation and exploitation in biosurfactant production. Biotechnol Lett 34:187–195. https://doi.org/10.1007/s10529-011-0782-6
Radzuan MN, Banat IM, Winterburn J (2017) Production and characterization of rhamnolipid using palm oil agricultural refinery waste. Biores Technol 225:99–105. https://doi.org/10.1016/j.biortech.2016.11.052
Ali Khan AH, Tanveer S, Alia S et al (2017) Role of nutrients in bacterial biosurfactant production and effect of biosurfactant production on petroleum hydrocarbon biodegradation. Ecol Eng 104:158–164. https://doi.org/10.1016/j.ecoleng.2017.04.023
Khan A, Rahman M, Zohora U et al (2011) Production of surfactin using pentose carbohydrate by Bacillus subtilis. J Environ Sci-China. https://doi.org/10.1016/S1001-0742(11)61079-6
Uzoigwe C, Burgess JG, Ennis CJ, Rahman PKSM (2015) Bioemulsifiers are not biosurfactants and require different screening approaches. Front Microbiol. https://doi.org/10.3389/fmicb.2015.00245
El-Sheshtawy HS, Aiad I, Osman ME et al (2015) Production of biosurfactant from Bacillus licheniformis for microbial enhanced oil recovery and inhibition the growth of sulfate reducing bacteria. Egypt J Pet 24:155–162. https://doi.org/10.1016/j.ejpe.2015.05.005
Leong T (2016) 16 - High-Power Ultrasonication for the Manufacture of Nanoemulsions and Nanodispersions. In: Knoerzer K, Juliano P, Smithers G (eds) Innovative Food Processing Technologies. Woodhead Publishing, pp 413–428
Sobrinho HBS, Rufino RD, Luna JM et al (2008) Utilization of two agroindustrial by-products for the production of a surfactant by Candida sphaerica UCP0995. Process Biochem 43:912–917. https://doi.org/10.1016/j.procbio.2008.04.013
Mcclements DJ (2007) Critical review of techniques and methodologies for characterization of emulsion stability. Crit Rev Food Sci Nutr 47:611–649. https://doi.org/10.1080/10408390701289292
Feng J, Shi Y, Yu Q et al (2016) Effect of emulsifying process on stability of pesticide nanoemulsions. Colloids Surf, A 497:286–292. https://doi.org/10.1016/j.colsurfa.2016.03.024
Giro MEA, Martins JJL, Rocha MVP et al (2009) Clarified cashew apple juice as alternative raw material for biosurfactant production by Bacillus subtilis in a batch bioreactor. Biotechnol J 4:738–747. https://doi.org/10.1002/biot.200800296
Martins PC, Martins VG (2018) Biosurfactant production from industrial wastes with potential remove of insoluble paint. Int Biodeterior Biodegradation 127:10–16. https://doi.org/10.1016/j.ibiod.2017.11.005
Chang Q (2016) Chapter 11 - Emulsion, Foam, and Gel. In: Chang Q (ed) Colloid and Interface Chemistry for Water Quality Control. Academic Press, pp 227–245
Morikawa M, Hirata Y, Imanaka T (2000) A study on the structure–function relationship of lipopeptide biosurfactants. Biochim et Biophys Acta (BBA)—Mol Cell Biol Lipids 1488:211–218. https://doi.org/10.1016/S1388-1981(00)00124-4
Varjani SJ, Upasani VN (2016) Carbon spectrum utilization by an indigenous strain of Pseudomonas aeruginosa NCIM 5514: production, characterization and surface active properties of biosurfactant. Biores Technol 221:510–516. https://doi.org/10.1016/j.biortech.2016.09.080
Acknowledgements
The authors would like to thank the Laboratory of Microbiology and Bioprocess Technology (Department of Chemical Engineering, Faculty of Industrial Technology, Institut Teknologi Bandung) for providing laboratory facilities and the Laboratory of Microbiology (School of Life Sciences and Technology, Institut Teknologi Bandung) for providing bacterial collection.
Funding
This study was supported by the Indonesian Agency for Agricultural Research and Development (Number: 602.4/Kpts/KP.320/H.1/7/2019) and the Ministry of Research and Technology of the Republic of Indonesia (Number: 2/E1/KP.PTNBH/2021).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. TS contributed to conceptualization, supervision, methodology, funding acquisition, and supervision. RSA contributed to writing-original draft, formal analysis, validations, investigations, project administration, and visualization. RP contributed to writing-review and editing, visualizations, supervision, conceptualization, and methodology. HH contributed to writing-review and editing, conceptualization, and methodology.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no relevant financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
The authors confirm that this manuscript has not been submitted to a preprint server before submitting it to Current Microbiology. This manuscript is original and has not been published simultaneously elsewhere in any form or language. The results presented in this manuscript are clear, honest, and without fabrication, falsification, or manipulation. The data, text, and theories by others are cited correctly.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adiandri, R.S., Purwadi, R., Hoerudin, H. et al. Evaluation of Biosurfactant Production by Bacillus Species Using Glucose and Xylose as Carbon Sources. Curr Microbiol 80, 250 (2023). https://doi.org/10.1007/s00284-023-03345-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03345-6