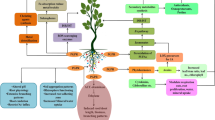
Abstract
Cadmium (Cd) is a heavy metal of considerable toxicity with destructive impacts on plants, microbes and environments. Its toxicity is due to mishandling and manual hazards in plants and is primarily observed within the soil to cause decline of plants and microbial activity inside the rhizosphere. Cadmium accumulation in crops and the probability of Cd entering the food chain are grave for public health in the worldwide. Cadmium toxicity leads to depletion in seed germination, initial seedling growth, plant biomass, chlorosis, necrosis, hindrance of photosynthetic machinery and other physiological and biological activities in plants. Cadmium triggers the reactive oxygen species (ROS) that influences gene mutation and DNA damage that affects the cell cycle and cell division. Cd toxicity altered the levels of phenolic compounds, carbohydrates, glycine betaine, proline and organic acids in crops. Under stress conditions, the plant growth promoting rhizobacteria (PGPR) have various properties such as enzymatic activities, plant growth hormones production, phosphate solubilization, siderophores production and chelating agents that help the plants tolerate against Cd stress and also increase phenolic compound levels and osmolytes. Hence, this review highlights the crucial role of cadmium tolerant PGPR for crop production, declining metal phytoavailability and enhancing morphological and physiological boundaries of plants under stress conditions. It could be an environment friendly and cost effective technology under sustainable crop production.

Similar content being viewed by others
Data Availability
Not applicable.
Code availability
Not applicable.
References
Gupta A, Gupta R, Singh RL (2017) Microbes and environment. Principles and applications of environmental biotechnology for a sustainable future. Springer Singapore India pp. 978–981 https://doi.org/10.1007/978-981-10-1866-4-3
Hinsinger P, Marschner P (2006) Rhizosphere perspectives and challenges a tribute to Lorenz Hiltner, Germany. Plant Soil 283:8–7. https://doi.org/10.1007/s11104-006-0057-5
Chibuike G, Obiora S (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci 2014:1–12. https://doi.org/10.1155/2014/752708
Zafar-Ul-Hye M, Naeem M, Danish S, Khan MJ, Fahad S, Datta R, Brtnicky M, Kintl A, Hussain GS, El-Esawi MA et al (2020) Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants (Basel) 9(10):1386. https://doi.org/10.3390/plants9101386
Garnier L, Simon PF, Thuleau P, Agnel JP, Blein JP et al (2006) Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ 29:1956–1969. https://doi.org/10.1111/j.1365-3040.2006.01571.x
Foyer CH, Noctor G (2013) Redox signaling in plants. Antioxi Redox Signal 18:2087–2090. https://doi.org/10.1089/ars.2013.5278
Jing YD, He ZL, Yang XE (2007) Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J Zhejiang Univ Sci B 8(3):192–207. https://doi.org/10.1631/jzus.2007.B0192
Sharma RK, Archana G (2016) Cadmium minimization in food crops by cadmium resistant plant growth promoting rhizobacteria. Appl Soil Ecol 107:66–78. https://doi.org/10.1016/j.apsoil.2016.05.009
Kumar A, Patel JS, Meena VS, Ramteke PW (2019) Plant growth-promoting rhizobacteria: strategies to improve abiotic stresses under sustainable agriculture. J Plant Nutri. https://doi.org/10.1080/01904167.2019.1616757
Hye MZ, Naeem M, Danish S, Khan MJ, Fahad S, Datta R, Brtnicky M, Kintl A, Hussain GS, El Esawi MA et al (2020) Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants 9:1386. https://doi.org/10.3390/plants9101386
Dong MF (2017) Inoculation of Fe/Mn-oxidizing bacteria enhances Fe/Mn plaque formation and reduces Cd and as accumulation in rice plant tissues. Ecotoxicol Environ Saf 144:507–513. https://doi.org/10.1007/s11104-016-2829-x
Nesler A (2017) Functional components of the bacterial CzcCBA efflux system reduce cadmium uptake and accumulation in transgenic tobacco plants. New Biotechnol 35:54–61. https://doi.org/10.1016/j.nbt.2016.11.006
Wang Q, Chen L, He LY, Sheng XF (2016) Increased biomass and reduced heavy metal accumulation of edible tissues of vegetable crops in the presence of plant growth-promoting Neorhizobium huautlense T1–17 and biochar. Agric Ecosys Environ 228:9–18
Vassilev A, Lidon F (2011) Cd-induced membrane damages and changes in soluble protein and free amino acid contents in young barley plants. Emirates J Food Agri. https://doi.org/10.9755/ejfa.v23i2.6347
Yang LP, Zhu J, Wang P, Zeng J, Tan R, Yang YZ, Liu ZM et al (2018) Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxi Environ Saf 160:10–18. https://doi.org/10.1016/j.ecoenv.2018.05.026
Huihui Z, Xin L, Zisong X, Yue W, Zhiyuan T, Meijunc A, Yuehui Z, Wenxu Z, Nan X, Guangyu S et al (2020) Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol Environ Saf 195:110469. https://doi.org/10.1016/j.ecoenv.2020.110469
Patsikka E, Marja K, Tyystjarvi E (2002) Excess copper predisposes photosystem II to photo inhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Am Soc Plant Physiol 21:834–847. https://doi.org/10.1104/pp.004788
Xie Y, Hu L, Du Z, Sun X, Amombo E et al (2014) Effects of cadmium exposure on growth and metabolic profile of bermudagrass [Cynodon dactylon (L.) Pers]. PLos ONE 9(12):e115279. https://doi.org/10.1371/journal.pone.0115279
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol 156:989–996. https://doi.org/10.1104/pp.111.175448
Jiang HM, Yang JC, Zhang JF (2007) Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ Pollut 147:750–756. https://doi.org/10.1016/j.envpol.2006.09.006
Qiu Q, Wang Y, Yang Z (2011) Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis) cultivars differing in cadmium accumulation. Food Chem Toxico 49(9):2260–2267. https://doi.org/10.1016/j.fct.2011.06.024
Vocciante M, Grifoni M, Fusini D, Petruzzelli G, Franchi E et al (2022) The role of plant growth-promoting rhizobacteria (PGPR) in mitigating plant’s environmental stresses. App Sci 12:1231. https://doi.org/10.5772/intechopen.87083
Umar W, Ayub MA, Rehman ur MZ, Ahmad HR, Farooqi ZUR, Shahzad A, Rehman U, Mustafa A, Nadeem M et al (2020) Nitrogen and phosphorus use efficiency in agroecosystems. In: Kumar S, Meena RS, Jhariya MK (eds) Resources use efficiency in agriculture. Springer, Singapore, pp 213–257. https://doi.org/10.1007/978-981-15-6953-1_7
Sureshbabu K, Amaresan N, Kumar K (2016) Amazing multiple function properties of plant growth promoting rhizobacteria in the rhizosphere soil. Int J Curr Microbiol App Sci 5(661):68. https://doi.org/10.20546/ijcmas.2016.502.074
Duffy BK, Défago G (1999) Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. App Environ Microbiol 65:2429–2438. https://doi.org/10.1128/AEM.65.6.2429-2438.1999
Kobayashi T, Nishizawa NK (2012) Iron uptake translocation, and regulation in higher plants. Ann Rev Plant Biol 63:131–152. https://doi.org/10.1146/annurev-arplant-042811-105522
Guo J, Muhammad H, Lv X, Wei T, Ren X, Jia H, Atif S, Hua L et al (2020) Prospects and applications of plant growth promoting rhizobacteria to mitigate soil metal contamination: a review. Chemo 246:125823. https://doi.org/10.1016/j.chemosphere.2020.125823
Singh P, Singh RK, Zhou Y, Wang J, Jiang Y, Shen N, Wang Y, Yang L, Jiang M et al (2022) Unlocking the strength of plant growth promoting Pseudomonas in improving crop productivity in normal and challenging environments: a review. J Plant Intera 17(1):220–238. https://doi.org/10.1080/17429145.2022.2029963
Asgher M, Khan MIR, Anjum NA, Khan NA (2015) Minimising toxicity of cadmium in plants role of plant growth regulators. Protoplasma 2523:399–413. https://doi.org/10.1007/s00709-014-0710-4
Spaepen S, Vanderleyden J (2011) Auxin and plant-microbe interactions. Cold Spring Harb Perspect Biol 3:1438. https://doi.org/10.1101/cshperspect.a001438
Shahid M, Javed MT, Mushtaq A, Akram MS, Mahmood F, Ahmed T, Noman M, Azeem M et al (2019) Microbe-mediated mitigation of cadmium toxicity in plants. In: Shahid M, Javed MT, Mushtaq A, Akram MS, Mahmood F, Ahmed T, Noman M, Azeem M (eds) Cadmium toxicity and tolerance in plants from physiology to remediation. Elsevier, Amsterdam, pp 427–449. https://doi.org/10.1016/B978-0-12-814864-8.00017-6
Karcz W, Kurtyka R (2007) Effect of cadmium on growth, proton extrusion and membrane potential in maize coleoptile segments. Bio Planta 51:713–719. https://doi.org/10.1007/s10535-007-0147-0
Hu YF, Zhou G, Na XF (2013) Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J Plant Physiol 170:965–975. https://doi.org/10.1016/j.jplph.2013.02.008
Zhou X, Zhang X, Ma C, Wu F, Jin X, Dini-Andreote F, Wei Z et al (2022) Biochar amendment reduces cadmium uptake by stimulating cadmium-resistant PGPR in the tomato rhizosphere. Chemos 307:136138. https://doi.org/10.1016/j.chemosphere.2022.136138
Rolon-Cardens GA, Arvizu-Gómez JL, Soria-Guerra RE, Pacheco-Aguilar JR, Alatorre-Cobos F, Hernández-Morales A et al (2022) The role of auxins and auxin-producing bacteria in the tolerance and accumulation of cadmium by plants. Environ Geochem Health. https://doi.org/10.1007/s10653-021-01179-4
Ghosh PK, Majumdar S (2022) Cadmium stress management in plants: prospects of plant growth-promoting rhizobacteria. In: Roy S, Mathur P, Chakraborty AP, Saha SP (eds) Plant stress: challenges and management in the new decade. Springer, Cham, pp 235–249. https://doi.org/10.1007/978-3-030-95365-2_15
Zafar-ul-Hye M, Danish S, Abbas M, Ahmad M, Munir T et al (2019) ACC deaminase producing PGPR Bacillus amyloliquefaciens and agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agronomy 9:343. https://doi.org/10.3390/agronomy9070343
Maxton A, Singh P, Masih SA (2018) ACC deaminase-producing bacteria mediated drought and salt tolerance in Capsicum annuum. J Plant Nutri 41(5):574–583. https://doi.org/10.1080/01904167.2017.1392574
Hassan W, Bashir S, Ali F, Ijaz M, Hussain M et al (2016) Role of ACC-deaminase and/or nitrogen fixing rhizobacteria in growth promotion of wheat (Triticum aestivum L.) under cadmium pollution. Environ Earth Sci 75:1–14. https://doi.org/10.1007/s12665-015-4902-9
Kamran M, Malik Z, Parveen A, Huang L, Riaz M et al (2020) Ameliorative effects of biochar on rapeseed (Brassica napus L.) growth and heavy metal immobilization in soil irrigated with untreated wastewater. J Plant Growth Regul 39:266–281. https://doi.org/10.1007/s00344-019-09980-3
Farid M, Shakoor MB, Ehsan S, Ali S, Zubair M et al (2013) Morphological, physiological and biochemical responses of different plant species to Cd stress. Intern J Chem and Biochem Sci 3:53–60
Huang H, Ullah F, Zhou DX, Yi M, Zhao Y et al (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800. https://doi.org/10.3389/fpls.2019.00800
Karuppanapandian T, Moon J, Kim C, Manoharan K, Kim W et al (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aus J crop sci 5:709–725. https://doi.org/10.3316/informit.282079847301776
Sandalio LM, Dalurzo HC, Gomez M, Romero PMC, LA Del R et al (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126. https://doi.org/10.1093/jexbot/52.364.2115
Somashekaraiah BV, Padmmaja K, Prasad ARK (1992) Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): involvement of lipid peroxides in chlorophyll degradation. Physio Plant 85:85–89. https://doi.org/10.1111/J.1399-3054
Okamoto OK, Pinto E, Latorre LR, Bechara EJH, Colepicolo P et al (2001) Antioxidant modulation in response to metal-induced oxidative stress in algal chloroplasts. Arch Environ Contam Toxicol 40:18–24. https://doi.org/10.1007/s002440010144
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K et al (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319. https://doi.org/10.1093/jxb/53.372.1305
Singh S, Khan NA, Nazar R, Anjum NA (2008) Photosynthetic traits and activities of antioxidant enzymes in Blackgram (Vigna mungo L. Hepper) under cadmium stress. Am J plant Physiol 3:25–32. https://doi.org/10.3923/ajpp.2008.25.32
Tran TA, Popova LP (2013) Functions and toxicity of cadmium in plants: recent advances and future prospects. Turk J Bot 37:1–13. https://doi.org/10.3906/bot-1112-16
Zulfqar U, Ayub A, Hussain S, Waraich EA, El-Esawi MA, Ishfaq M, Ahmad M, Ali N, Maqsood MF et al (2021) Cadmium toxicity in plants: recent progress on morpho-physiological effects and remediation strategies. J Soil Sci Plant Nutri 22:212–269. https://doi.org/10.1007/s42729-021-00645-3
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Haluskova L, Valentovicova K, Huttova J, Mistrik I, Tamas L et al (2009) Effect of abiotic stresses on glutathione peroxidase and glutathione S-transferase activity in barley root tips. Plant Physiol Biochem 47:1069–1074. https://doi.org/10.1016/j.plaphy.2009.08.003
Bano A, Gupta A, Rai S, Fatima T, Sharma S et al (2021) Mechanistic role of reactive oxygen species and its regulation via the antioxidant system under environmental stress. Chapter Plant stress Physio. https://doi.org/10.5772/intechopen.101045
Bashir K, Nagasaka S, Itai RN, Kobayashi T, Takahashi M et al (2007) Expression and enzyme activity of glutathione reductase is upregulated by Fe-deficiency in graminaceous plants. Plant Mol Biol 65:277–284. https://doi.org/10.1007/s11103-007-9216-1
Hojati M, Modarres-Sanavy SAM, Karimi M, Ghanati F (2010) Responses of growth and antioxidant systems in Carthamus tinctorius L. under water deficit stress. Acta Physiol Plant 33:105–112. https://doi.org/10.1007/s11738-010-0521-y
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Tren Plant Sci 7:405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
El-beltagi HS, Mohamed HI (2013) Alleviation of cadmium toxicity in Pisum sativum L. seedlings by calcium chloride. Notu Botanic Horti Agrobota Cluj-Napoca 41(1):157–168. https://doi.org/10.15835/nbha4118910
Lavid N, Schwartz A, Lewinsohn E, Tel-Or E (2001) Phenols and phenol oxidases are involved in cadmium accumulation in the water plants Nymphoides peltate (Menyanthaceae) and Nymphaeae (Nymphaeaceae). Planta 214(2):189–195. https://doi.org/10.1007/s004250100610
Zheng G, Lv HP, Gao S, Wang SR (2010) Effects of cadmium on growth and antioxidant responses in Glycyrrhiza uralensis seedlings. Plant Soil and Environ 56:08–515. https://doi.org/10.17221/30/2010-PSE
Kieffera P, Schröder P, Dommes J, Hoffmanna L, Renauta J, Hausman JF et al (2009) Proteomic and enzymatic response of poplar to cadmium stress. J Proteomic 72(3):379–396. https://doi.org/10.1016/j.jprot.2009.01.014
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11. https://doi.org/10.1093/jexbot/53.366.1
Manzoor M (2019) Metal tolerance of arsenic-resistant bacteria and their ability to promote plant growth of Pteris vittata in Pb contaminated soil. Sci Tot Environ 660:18–24. https://doi.org/10.1016/j.scitotenv.2019.01.013
Khanna K, Jamwal VL, Gandhi SG, Ohri P, Bhardwaj R et al (2019) Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci Report 9:5855. https://doi.org/10.1038/s41598-019-41899-3
Dalcorso G, Farinati S, Furini A (2010) Regulatory networks of cadmium stress in plants. Plant Signal Behav 5:663–667. https://doi.org/10.4161/psb.5.6.11425
Lee K, Bae DW, Kim SH, Han HJ, Liu X, Park HC, Lim CO, Lee SY, Chung WS et al (2010) Comparative proteomic analysis of the short-term responses of rice roots and leaves to cadmium. J Plant Physio 167:161–168. https://doi.org/10.1016/j.jplph.2009.09.006
Shen ZG, Zhao FJ, McGrath SP (1997) Uptake and transport of zinc in the hyper accumulator Thlaspi caerulescens and the non-hyperacumulator Thlaspi ochroleucum. Plant Cell Environ 20:898–906. https://doi.org/10.1046/j.1365-3040.1997
Kang SM, Radhakrishnan R, You YH, Khan AL, Lee KE et al (2015) Enterobacter asburiae KE 17 association regulates physiological changes and mitigates the toxic effects of heavy metals in soybean. Plant Biol 17:1013–1022. https://doi.org/10.1111/plb.12341
Parmar P, Kumari N, Sharma V (2013) Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot Stud 54:45. https://doi.org/10.1186/1999-3110-54-45
Kamran MA, Syed JH, Eqani SAMAS, Munis MFH, Chaudhary HJ et al (2015) Effect of plant growth-promoting rhizobacteria inoculation on cadmium (Cd) uptake by Eruca sativa. Environ Sci Pol Res 22:9275–9283. https://doi.org/10.1007/s11356-015-4074-x
Khanna K, Jamwal VL, Gandhi SG, Ohri P, Bhardwaj R et al (2018) Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci Rep 9:5855. https://doi.org/10.1038/s41598-019-41899-3
Li J, Cao X, Jia X, Liu L, Cao H, Qin W, Li M et al (2021) Iron deficiency leads to chlorosis through impacting chlorophyll synthesis and nitrogen metabolism in Areca catechu L. Front Plant Sci 12:710093. https://doi.org/10.3389/fpls.2021.710093
Mendoza CDG, Butko E, Springer F, Torpey J, Komives E, Kehr J, Schroeder J et al (2008) Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J 54:249–259. https://doi.org/10.1111/j.1365-313X.2008.03410.x
Verbruggen N, Hermans C, Schat H (2009) Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 181:759–776. https://doi.org/10.1111/j.1469-8137.2008.02748.x
Kuhnlenz T, Schmidt H, Uraguchi S, Clemens S (2014) Arabidopsis thaliana phytochelatin synthase 2 is constitutively active in vivo and can rescue the growth defect of the PCS1-deficient cad1-3 mutant on Cd-contaminated soil. J Exp Bot 65:4241–4253. https://doi.org/10.1093/jxb/eru195
Chen J, Yang L, Gu J, Bai X, Ren Y et al (2015) MAN3 gene regulates cadmium tolerance through the glutathione dependent pathway in Arabidopsis thaliana. New Phytol 205:570–582. https://doi.org/10.1111/nph.13101
Song, (2017) A cadmium stress-responsive gene AtFC1 confers plant tolerance to cadmium toxicity. BMC Plant Bio 1:187. https://doi.org/10.1186/s12870-017-1141-0
Rascio N, Navari IF (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–181. https://doi.org/10.1016/j.plantsci.2010.08.016
Mei H, Hirschi KD, Cheng NH, Zhao J, Park S, Escareno RA, Pittman JK et al (2009) Root development under metal stress in Arabidopsis thaliana requires the H+/cation antiporter CAX4. New Phytol 183:95–105. https://doi.org/10.1111/j.1469-8137.2009.02831.x
Hasan MK, Cheng Y, Kanwar MK, Chu XY, Ahammed GJ et al (2017) Responses of plant proteins to heavy metal stress: a review. Front Plant Sci 8:1–16. https://doi.org/10.3389/fpls.2017.01492
Espinas NA, Kobayashi K, Sato Y, Mochizuki N, Takahashi K et al (2016) Allocation of heme is differentially regulated by ferrochelatase isoforms in Arabidopsis cells. Front Plant Sci 7:1326. https://doi.org/10.3389/fpls.2016.01326
Raza A, Habib M, Kakavand SN, Zahid Z, Zahra N, Sharif R, Hasanuzzaman M et al (2020) Phytoremediation of cadmium: physiological, biochemical, and molecular mechanisms. Biol 9:177. https://doi.org/10.3390/biology9070177
Ahmadpour P, Soleimani M (2015) Cadmium accumulation and translocation in Jatropha curcas grown in contaminated soils. JWSS-Isfahan Uni Technol 19:179–190
Naveed M, Mustafa A, Majeed S, Naseem Z, Saeed Q, Khan A, Nawaz A, Baig KS, Chen JT et al (2020) Enhancing cadmium tolerance and pea plant health through enterobacter sp. MN17 inoculation together with biochar and gravel sand. Plants 9:530. https://doi.org/10.3390/plants9040530
Pramanik K, Mitra S, Sarkar A, Soren T, Maiti TK et al (2017) Characterization of cadmium-resistant Klebsiella pneumoniae MCC 3091 promoted rice seedling growth by alleviating phytotoxicity of cadmium. Env Sci Pollut Res 24:24419–24437. https://doi.org/10.1007/s11356-017-0033-z
Mitra S, Pramanik K, Sarkar A, Ghosh PK, Soren T, Maiti TK et al (2018) Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol Environ Safety 156:183–196. https://doi.org/10.1016/j.ecoenv.2018.03.001
Ahmad I, Akhtar MJ, Asghar HN, Ghafoor U, Shahid M et al (2016) Differential effects of plant growth-promoting rhizobacteria on maize growth and cadmium uptake. J Plant Growth Regul 35:303–315. https://doi.org/10.1007/s00344-015-9534-5
Eriksson J, Ingrid O (2007) Cadmium concentration in winter wheat as affected by nitrogen fertilizer. Eur J Agro 26:209–214. https://doi.org/10.1016/j.eja.2006.09.010
Wani PA, Khan MS (2010) Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem Toxicol 48:3262–3267. https://doi.org/10.1016/j.fct.2010.08.035
Mahdi I, Fahsi N, Hafidi M, Benjelloun S, Allaoui A, Biskri L et al (2021) Rhizospheric phosphate solubilizing Bacillus atrophaeus GQJK17 S8 increases quinoa seedling, withstands heavy metals, and mitigates salt stress. Sustainability 13:3307. https://doi.org/10.3390/su13063307
Prapagdee B, Chanprasert M, Mongkolsuk S (2013) Bioaugmentation with cadmium-resistant plant growth-promoting rhizobacteria to assist cadmium phytoextraction by Helianthus annuus. Chemosphere 92(6):659–66. https://doi.org/10.1016/j.chemosphere.2013.01.082
Pal AK, Sengupta C (2019) Isolation of cadmium and lead tolerant plant growth promoting rhizobacteria: Lysinibacillus varians and pseudomonas putida from indian agricultural soil. Soil Sedim Contam: An Intern J 28(7):601–629. https://doi.org/10.1080/15320383.2019.1637398
Pramanik K, Mitra S, Sarkar A, Maiti TK (2018) Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. J Hazar Mater 351:317–329. https://doi.org/10.1016/j.jhazmat.2018.03.009
González O, Ortíz CR, Díaz PC, Díaz PAL, Magaña DV et al (2017) Non-ribosomal peptide synthases from Pseudomonas aeruginosa play a role in cyclodipeptide biosynthesis, quorum Sensing regulation, and root development in a plant host. Microb Ecol 73:616–629. https://doi.org/10.1007/s00248-016-0896-4
Zafar-ul-Hye M, Shahjahan A, Danish S, Abid M, Qayyum MF et al (2018) Mitigation of cadmium toxicity induced stress in wheat by ACC-deaminase containing PGPR isolated from cadmium polluted wheat rhizosphere. Pak J Bot 50(5):1727–1734
Ghavami N, Alikhani HA, Pourbabaei AA, Besharati H (2017) Effects of two new siderophore-producing rhizobacteria on growth and iron content of maize and canola plants. J Plant Nutri 40(5):736–746. https://doi.org/10.1080/01904167.2016.1262409
Maslennikova D, Nasyrova K, Chubukova O, Akimova E, Baymiev A, Blagova D, Ibragimov A, Lastochkina O et al (2022) Effects of Rhizobium leguminosarum Thy2 on the growth and tolerance to cadmium stress of wheat plants. Life 12:1675. https://doi.org/10.3390/life12101675
Patel M, Patel K, Al-Keridis LA, Alshammari N, Badraoui R, Elasbali AM, Al-Soud WA, Hassan MI, Yadav DK, Adnan M et al (2022) Cadmium-tolerant plant growth-promoting bacteria Curtobacterium oceanosedimentum improves growth attributes and strengthens antioxidant system in chili (Capsicum frutescens). Sustain 14:4335. https://doi.org/10.3390/su14074335
Liaquat F, Munis MFH, Arif S (2020) Cd-tolerant SY-2 strain of Stenotrophomonas maltophilia: a potential PGPR, isolated from the Nanjing mining area in China. 3 Biotech 10:519. https://doi.org/10.1007/s13205-020-02524-7
Ganesan V (2008) Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizopseudomonad. Curr Microbiol 56:403–407. https://doi.org/10.1007/s00284-008-9099-7
Acknowledgements
All authors are thankful to Professor In-charge, Rajiv Gandhi South Campus, Banaras Hindu University, Barkachha, Mirzapur, Uttar Pradesh, India, for providing the necessary facilities required for conducting the review work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualizations: AK, NK, AS, Writing—original draft, review and editing: DK, DKY, AV, NS. All authors have study and agreed the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethics Approval
Not applicable.
Consent to Participate
All the subjects signed the informed consent to participate in the study.
Consent for Publication
We, the undersigned, give our consent for the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, A., Kumari, N., Singh, A. et al. The Effect of Cadmium Tolerant Plant Growth Promoting Rhizobacteria on Plant Growth Promotion and Phytoremediation: A Review. Curr Microbiol 80, 153 (2023). https://doi.org/10.1007/s00284-023-03267-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03267-3