Abstract
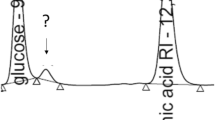
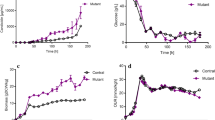
Precursor engineering is an effective strategy for the overproduction of secondary metabolites. The polyene macrolide rimocidin, which is produced by Streptomyces rimosus M527, exhibits a potent activity against a broad range of phytopathogenic fungi. It has been predicted that malonyl-CoA is used as extender units for rimocidin biosynthesis. Based on a systematic analysis of three sets of time-series transcriptome microarray data of S. rimosus M527 fermented in different conditions, the differentially expressed accsr gene that encodes acetyl-CoA carboxylase (ACC) was found. To understand how the formation of rimocidin is being influenced by the expression of the accsr gene and by the concentration of malonyl-CoA, the accsr gene was cloned and over-expressed in the wild-type strain S. rimosus M527 in this study. The recombinant strain S. rimosus M527-ACC harboring the over-expressed accsr gene exhibited better performances based on the enzymatic activity of ACC, intracellular malonyl-CoA concentrations, and rimocidin production compared to S. rimosus M527 throughout the fermentation process. The enzymatic activity of ACC and intracellular concentration of malonyl-CoA of S. rimosus M527-ACC were 1.0- and 1.5-fold higher than those of S. rimosus M527, respectively. Finally, the yield of rimocidin produced by S. rimosus M527-ACC reached 320.7 mg/L, which was 34.0% higher than that of S. rimosus M527. These results confirmed that malonyl-CoA is an important precursor for rimocidin biosynthesis and suggested that an adequate supply of malonyl-CoA caused by accsr gene over-expression led to the improvement in rimocidin production.




Similar content being viewed by others
References
Liu R, Deng Z, Liu T (2018) Streptomyces species: ideal chassis for natural product discovery and overproduction. Metab Eng 50:74–84
Kemung HM, Tan LT, Khan TM et al (2018) Streptomyces as a prominent resource of future anti-MRSA drugs. Front Microbiol 9:2221
Quinn GA, Banat AM, Abdelhameed AM et al (2020) Streptomyces from traditional medicine: sources of new innovations in antibiotic discovery. J Med Microbiol 69:1040–1048
Zhang LX, Demain AL (2005) Natural products: drug discovery and therapeutic medicine. The Humana Press, Totowa
Jung W, Kim E, Yoo YJ et al (2014) Characterization and engineering of the ethylmalonyl-CoA pathway towards the improved heterologous production of polyketides in Streptomyces venezuelae. Appl Microbiol Biotechnol 98(8):3701–3713
Li L, Liu X, Jiang W et al (2019) Recent advances in synthetic biology approaches to optimize production of bioactive natural products in Actinobacteria. Front Microbiol 10:2467
Palazzotto E, Tong Y, Lee SY et al (2019) Synthetic biology and metabolic engineering of actinomycetes for natural product discovery. Biotechnol Adv 37(6):107366
Blažič M, Kosec G, Baebler Š et al (2015) Roles of the crotonyl-CoA carboxylase/reductase homologues in acetate assimilation and biosynthesis of immunosuppressant FK506 in Streptomyces tsukubaensis. Microb Cell Fact 14:164
Jin XM, Chang YK, Lee JH et al (2017) Effects of increased NADPH concentration by metabolic engineering of the pentose phosphate pathway on antibiotic production and sporulation in Streptomyces lividans TK24. J Microbiol Biotechnol 27(10):1867–1876
Li S, Li Z, Pang S et al (2021) Coordinating precursor supply for pharmaceutical polyketide production in Streptomyces. Curr Opin Biotechnol 69:26–34
Liu H, Reynolds KA (2001) Precursor supply for polyketide biosynthesis: the role of crotonyl-CoA reductase. Metab Eng 3(1):40–48
Olano C, Lombó F, Méndez C et al (2008) Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab Eng 10(5):281–292
Wilson MC, Moore BS (2012) Beyond ethylmalonyl-CoA: the functional role of crotonyl-CoA carboxylase/reductase homologs in expanding polyketide diversity. Nat Prod Rep 29(1):72–86
Hertweck C (2009) The biosynthetic logic of polyketide diversity. Angew Chem Int Ed Engl 48(26):4688–4716
Wei J, Zhang Y, Yu T et al (2016) A unified molecular mechanism for the regulation of acetyl-CoA carboxylase by phosphorylation. Cell Discov 2:16044
Milke L, Marienhagen J (2020) Engineering intracellular malonyl-CoA availability in microbial hosts and its impact on polyketide and fatty acid synthesis. Appl Microbiol Biotechnol 104(14):6057–6065
Yu Z, Lv H, Wu Y et al (2019) Enhancement of FK520 production in Streptomyces hygroscopicus by combining traditional mutagenesis with metabolic engineering. Appl Microbiol Biotechnol 103(23–24):9593–9606
Ryu YG, Butler MJ, Chater KF et al (2006) Engineering of primary carbohydrate metabolism for increased production of actinorhodin in Streptomyces coelicolor. Appl Environ Microbiol 72(11):7132–7139
Sowiński P, Pawlak J, Borowski E et al (1995) Stereostructure of rimocidin. J Antibiot (Tokyo) 48(11):1288–1291
Seco EM, Pérez-Zúñiga FJ, Rolón MS et al (2004) Starter unit choice determines the production of two tetraene macrolides, rimocidin and CE-108, in Streptomyces diastaticus var. 108. Chem Biol 11(3):357–366
Jeon BJ, Kim JD, Han JW et al (2016) Antifungal activity of rimocidin and a new rimocidin derivative BU16 produced by Streptomyces mauvecolor BU16 and their effects on pepper anthracnose. J Appl Microbiol 120(5):1219–1228
Song Z, Liao Z, Hu Y et al (2019) Development and optimization of an intergeneric conjugation system and analysis of promoter activity in Streptomyces rimosus M527. J Zhejiang Univ Sci B 20(11):891–900
Song Z, Ma Z, Bechthold A et al (2020) Effects of addition of elicitors on rimocidin biosynthesis in Streptomyces rimosus M527. Appl Microbiol Biotechnol 104(10):4445–4455
Liao Z, Song Z, Xu J et al (2020) Identification of a gene from Streptomyces rimosus M527 negatively affecting rimocidin biosynthesis and morphological differentiation. Appl Microbiol Biotechnol 104(23):10191–10202
Zhao Y, Song Z, Ma Z et al (2019) Sequential improvement of rimocidin production in Streptomyces rimosus M527 by introduction of cumulative drug-resistance mutations. J Ind Microbiol Biotechnol 46(5):697–708
Zhao Y, Lu D, Bechthold A et al (2018) Impact of otrA expression on morphological differentiation, actinorhodin production, and resistance to aminoglycosides in Streptomyces coelicolor M145. J Zhejiang Univ Sci B 19(9):708–717
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Kieser T, Bibb MJ, Buttner MJ et al (2000) Practical Streptomyces genetics: a laboratory manual. John Innes Foundation, Norwich
Pierangeli SS, Harris EN (2018) A protocol for determination of anticardiolipin antibodies by ELISA. Nat Protoc 3(5):840–848
Arabolaza A, Shillito ME, Lin TW et al (2010) Crystal structures and mutational analyses of acyl-CoA carboxylase beta subunit of Streptomyces coelicolor. Biochemistry 49(34):7367–7376
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Escudero L, Al-Refai M, Nieto C et al (2015) New rimocidin/CE-108 derivatives obtained by a crotonyl-CoA carboxylase/reductase gene disruption in Streptomyces diastaticus var. 108: substrates for the polyene carboxamide synthase PcsA. PLoS ONE 10(8):e0135891
Tippelt A, Nett M (2021) Saccharomyces cerevisiae as host for the recombinant production of polyketides and nonribosomal peptides. Microb Cell Fact 20(1):161
Ren J, Cui Y, Zhang F et al (2014) Enhancement of nystatin production by redirecting precursor fluxes after disruption of the tetramycin gene from Streptomyces ahygroscopicus. Microbiol Res 169(7–8):602–608
Pérez-Zúñiga FJ, Seco EM, Cuesta T et al (2004) CE-108, a new macrolide tetraene antibiotic. J Antibiot (Tokyo) 57(3):197–204
Caffrey P, Aparicio JF, Malpartida F et al (2008) Biosynthetic engineering of polyene macrolides towards generation of improved antifungal and antiparasitic agents. Curr Top Med Chem 8(8):639–653
Funding
This work was supported by the National Natural Science Foundation of China (31772213, 31972320), the Zhejiang Province Natural Science Foundation (LY20C140006), and the Key Program of Zhejiang Province Natural Science Foundation (LZ22C140002).
Author information
Authors and Affiliations
Contributions
ZJL, JYZ, and YS conducted experiments. ZM and YYZ designed the experiments and wrote this article. AB and XPY checked the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liao, Z., Zhang, J., Shi, Y. et al. Improvement of Rimocidin Biosynthesis by Increasing Supply of Precursor Malonyl-CoA via Over-expression of Acetyl-CoA Carboxylase in Streptomyces rimosus M527. Curr Microbiol 79, 174 (2022). https://doi.org/10.1007/s00284-022-02867-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02867-9