Abstract
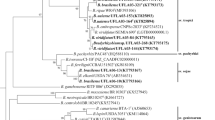
Bradyrhizobium is a genus of plant growth-promoting rhizobacteria (PGPR) that have been studied for several decades mainly for the ability to fix diazotrophic nitrogen after having been established endosymbiotically inside root nodules of the legumes of Fabaceae. The aim of this work was to evaluate the capability of Bradyrhizobium to promote the growth of crops belonging to other families, in this case, rice (Oryza sativa), both in laboratory and in field trials. For laboratory test, surface-sterilized rice seeds were soaked with cultures of each strain and planted in pots. Plant length and dry weight were measured after 35 days. For the field test, rice seeds of varieties Yeruá La Plata and Gurí INTA were inoculated with the three best strains observed in the laboratory test and planted in plots. After 60 days of growth, plant length and dry weight were measured. At harvest time, we measured the dry weight of the aerial part, yield and thousand-grain weight. Inoculation with any of the three species described provoked significant increments compared to the uninoculated control at least in one of the parameters measured, both in the laboratory and in the field tests. Bradyrhizobium japonicum E109 was the strain that promoted rice growth the most in the lab while Bradyrhizobium elkanii SEMIA 587 was the strain that promoted rice growth the most in the field, with increments in yield of approximately 1000 kg/ha. Data obtained suggest that the Bradyrhizobium species promoted all rice growth and yield.





Similar content being viewed by others
References
Gerland P, Raftery AE, Ševčíková H, Li N, Gu D, Spoorenberg T, Alkema L, Fosdick BK, Chunn J, Lalic N (2014) World population stabilization unlikely this century. Science 346:234–237. https://doi.org/10.1126/science.1257469
Liu X, Wang H, Zhou J, Hu F, Zhu D, Chen Z (2016) Effect of N fertilization pattern on rice yield, N use efficiency and fertilizer–N fate in the Yangtze river basin China. PLoS ONE 11:e0166002. https://doi.org/10.1371/journal.pone.0166002
Jacoby R, Peukert M, Succurro A, Koprivova A, Kopriva S (2017) The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front Plant Sci 8:1617. https://doi.org/10.3389/fpls.2017.01617
Paungfoo-Lonhienne C, Visser J, Lonhienne TGA, Schmidt S (2012) Past, present and future of organic nutrients. Plant Soil 359:1–18. https://doi.org/10.1007/s11104-012-1357
Van der Heijden MGA, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310. https://doi.org/10.1111/j.1461-0248.2007.01139.x
Verbon EH, Liberman LM (2016) Beneficial microbes affect endogenous mechanisms controlling root development. Trends Plant Sci 21:218–229. https://doi.org/10.1016/j.tplants.2016.01.013
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37:634–663. https://doi.org/10.1111/1574-6976.12028
McNear DH Jr (2013) The rhizosphere-roots, soil and everything in between. Nat Educ Knowl 4:1
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327. https://doi.org/10.1007/s11274-011-0979-9
Pathania N, Gosal SK, Saroa GS, Vikal Y (2014) Molecular characterization of diazotrophic bacteria isolated from rhizosphere of wheat cropping system from central plain region of Punjab. Afr J Microbiol Res 8:862–871. https://doi.org/10.5897/AJMR2013.5948
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571. https://doi.org/10.1023/A:1026037216893
Rodriguez-Navarro DN, Oliver IM, Contreras MA, Ruiz-Sainz JE (2010) Soybean interactions with soil microbes, agronomical and molecular aspects. Agron Sustain Dev 31:173–190. https://doi.org/10.1051/agro/2010023
Jordan D (1982) NOTES: transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow growing, root nodule bacteria from leguminous plants. Int J Syst Bacteriol 32:136–139
Kuykendall LD, Saxena B, Devine TE, Udell SE (1992) Genetic diversity in Bradyrhizobium japonicum and a proposal for Bradyrhizobium elkanii sp. nov. Can J Microbiol 38:501–505
Marçon Delamuta JR, Ribeiro RA, Ormeño-Orrillo E, Soares Melo I, Martínez-Romero E, Hungria M (2013) Polyphasic evidence supporting the reclassification of Bradyrhizobium japonicum group Ia strains as Bradyrhizobium diazoefficiens sp. nov. Int J Syst Evol Microbiol 63:3342–3351. https://doi.org/10.1099/ijs.0.049130-0
Siqueira et al. (2014). Comparative genomics of Bradyrhizobium japonicum CPAC 15 and Bradyrhizobium diazoefficiens CPAC 7: elite model strains for understanding symbiotic performance with soybean. BMC Genomics 15:420. https://www.biomedcentral.com/1471-2164/15/420.
Halverson LJ, Stacey G (1986) Signal exchange in plant-microbe interactions. Microbiol Rev 50:193–225
Sadowsky MJ, Tully RE, Cregan PB, Keyser HH (1987) Genetic diversity in Bradyrhizobium japonicum Serogroup 123 and its relation to genotype-specific nodulation of soybean. Appl Environ Microbiol 53:2624–2630
Itakura M, Saeki K, Omori H, Yokoyama T, Kaneko T, Tabata S, Ohwada T, Tajima S, Uchiumi T, Honnma K, Fujita K, Iwata H, Saeki Y, Hara Y, Ikeda S, Eda S, Mitsui H, Minamisawa K (2009) Genomic comparison of Bradyrhizobium japonicum strains with different symbiotic nitrogen-fixing capabilities and other Bradyrhizobiaceae members. ISME J 3:326–339. https://doi.org/10.1038/ismej.2008.88
Antoun H, Beauchamp CJ, Goussard N, Chabot R, Lalande R (1998) Potential of Rhizobium and Bradyrhizobium species as plant growth-promoting rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.). In: Hardarson G, Broughton WJ (eds) Molecular microbial ecology of the soil. Developments in plant and soil sciences. vol 83, Springer, Dordrecht
Chebotar VK, Asis CA Jr, Akao S (2001) Production of growth-promoting substances and high colonization ability of rhizobacteria enhance the nitrogen fixation of soybean when coinoculated with Bradyrhizobium japonicum. Biol Fertil Soils 34:427–432. https://doi.org/10.1007/s00374-001-0426-4
Tan Z, Hurek T, Vinuesa P, Müller P, Ladha JK, Reinhold-Hurek B (2001) Specific detection of Bradyrhizobium and Rhizobium strains colonizing rice (Oryza sativa) roots by 16S–23S ribosomal DNA intergenic spacer-targeted PCR. Appl Environ Microb 67:3655–3664. https://doi.org/10.1128/AEM.67.8.3655-3664.2001
Chaintreuil C, Giraud E, Prin Y, Lorquin J, Bâ A, Gillis M, de Lajudie P, Dreyfus B (2000) Photosynthetic bradyrhizobia are natural endophytes of the African wild rice Oryza breviligulata. Appl Environ Microbiol 66(12):5437–5447
Mano H, Morisaki H (2008) Endophytic bacteria in the rice plant. Microbes Environ 23:109–117. https://doi.org/10.1264/jsme2.109
Sreevidya VS, Hernandez-Oane RJ, So RB, Sullia SB, Stacey G, Ladha JK, Reddy PM (2005) Expression of the legume symbiotic lectin genes psl and gs52 promotes rhizobial colonization of roots in rice. Plant Sci 169:726–736. https://doi.org/10.1016/j.plantsci.2005.05.024
Vincent JM (1970) A manual for the practical study of the root-nodule bacteria. Blackwell, Oxford
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Sumanta N, Haque CI, Jaishee N, Roy S (2014) Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res J Chem Sci 4:63–69
Gaby JC, Buckley DH (2012) A comprehensive evaluation of PCR primers to amplify the nifH gene of nitrogenase. PLoS ONE 7:e42149. https://doi.org/10.1371/journal.pone.0042149
Piromyou P, Greetatorn T, Teamtisong K, Tittabutr P, Boonkerd N, Teaumroong N (2017) Potential of rice stubble as a reservoir of Bradyrhizobial inoculum in rice-legume crop rotation. Appl Environ Microbiol 83:e01488-e1517. https://doi.org/10.1128/AEM.01488-17
Filella I, Serrano L, Serra J, Penuelas J (1995) Evaluating wheat nitrogen status with canopy reflectance indices and discriminant analysis. Crop Sci 35:1400–1405
Moran JA, Mitchell AK, Goodmanson G (2000) Differentiation among effects of nitrogen fertilization treatments on conifer seedlings by foliar reflectance: a comparison of methods. Tree Physiol 20:1113–1120
Brown SB, Houghton JD, Hendry GAF (1991) Chlorophyll breakdown. In: Scheer H (ed) Chlorophylls. CRC Press, Boca Raton, FL, pp 465–489
Costache MA, Campeanu G, Neata G (2012) Studies concerning the extraction of chlorophyll and total carotenoids from vegetables. Rom Biotechnol Lett 17:7702–7708
Buckland SM, Price AH, Hendry GAF (1991) The role of ascorbate in drought-treated Cochlearia atlantica Pobed. and Armeria maritima (Mill.) Willd. New Phytol 119:155–160
Vicaş SI, Laslo V, Pantea S, Bandici GE (2010) Chlorophyll and carotenoids pigments from mistletoe (Viscum album) leaves using different solvents. Analele Universităţii din Oradea Fascicula Biologie 2:213–218.
Hung PQ, Annapurna K (2004) Isolation and characterization of endophytic bacteria in soybean (Glycine sp.). Omonrice 12:92–101
Kobayashi D, Palumbo JD (2000) Bacterial endophytes and their effects on plants and uses in agriculture. In: Bacon CW, White JF (eds) Microbial endophytes. Marcel Dekker, New York, pp 199–233
Liu L, Kloepper JW, Tuzun S (1995) Induction of systemic resistance in cucumber against Fusarium wilt by plant growth-promoting rhizobacteria. Phytopathology 85:695–698
Sturz A, Matheson B (1996) Populations of endophytic bacteria which influence host-resistance to Erwinia-induced bacterial soft rot in potato tubers. Plant Soil 184:265–271
Kirchhof G, Reis VM, Baldani JI, Eckert B, Döbereiner J, Hartmann A (1997) Occurrence, physiological and molecular analysis of endophytic diazotrophic bacteria in gramineous energy plants. In: Ladha JK et al (eds) Opportunities for biological nitrogen fixation in rice and other non-legumes. Developments in plant and soil sciences. vol 75, Springer, Dordrecht, pp 45–55
Reinhold-Hurek B, Hurek T (1998) Life in grasses: diazotrophic endophytes. Trends Microbiol 6:139–144
Sturz AV, Christie BR, Matheson BG, Nowak J (1997) Biodiversity of endophytic bacteria which colonize red clover nodules, roots, stems and foliage and their influence on host growth. Biol Fertil Soil 25:13–19
Devine TE, Kuykendall LD, O’Neill JJ (1988) DNA homology group and the identity of Bradyrhizobial strains producing rhizobitoxine-induced foliar chlorosis on soybean. Crop Sci 28:938–939
Sessitsch A, Mitter B (2015) 21st century agriculture: integration of plant microbiomes for improved crop production and food security. Microb Biotechnol 8:32–33. https://doi.org/10.1111/1751-7915.12180
Mbai FN, Magiri EN, Matiru VN, Ng’ang’a J, Nyambati VCS (2013) Isolation and characterisation of bacterial root endophytes with potential toenhance plant growth from Kenyan Basmati rice. Am Int J Contemp Res 3:25–40
Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Martinez-Viveros O, Jorquera MA, Crowley DE, Gajardo G, Mora ML (2010) Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nutr 10:293–319
Ai’shah ON, Amir HG, Keng CL, Othman AR (2010) Influence of various combinations of diazotrophs and chemical N fertilizer on plant growth and N2 fixation capacity of oil palm seedlings (Elaeis guineensis Jacq.). Thai J Agric Sci 42:139–149
Acknowledgements
D.P. was supported by a grant from National Science Foundation, Colombo, Sri Lanka. J.M.R. was supported by a grant from the International Centre for Genetic Engineering and Biotechnology (ICGEB), Buenos Aires, Argentina. P.A.B. is a scientist supported by the Comisión de Investigaciones Científicas de la Provincia de Buenos Aires. This research was also possible thanks to funds made available by ICGEB and the support from the Faculty of Agriculture, University of Ruhuna, Mapalana, Kamburupitiya, Sri Lanka, and Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata (FCAyF).
Author information
Authors and Affiliations
Contributions
DP performed the experiments in the laboratory; JMR and RB followed the field trials; SG, PAB and GD planned the experiments, elaborated the results and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Padukkage, D., Geekiyanage, S., Reparaz, J.M. et al. Bradyrhizobium japonicum, B. elkanii and B. diazoefficiens Interact with Rice (Oryza sativa), Promote Growth and Increase Yield. Curr Microbiol 78, 417–428 (2021). https://doi.org/10.1007/s00284-020-02249-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02249-z