Abstract
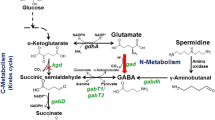
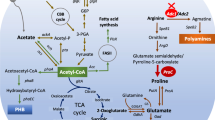
GABA accumulation and glutamate decarboxylase (GAD) activity, the principal enzyme involved in GABA formation, was investigated in cyanobacterium Synechocystis sp. PCC 6803 wild-type (WT) and gad knockout mutant strains grown in medium containing different nitrogenous compounds. Nitrate was the best nitrogen source for GAD activity and GABA accumulation followed by nitrite, ammonium, and urea. An increase in the accumulation of GABA was observed in WT and mutant cells grown for 24 h in medium supplemented with 0.5 mM putrescine or spermidine with a parallel increase in GAD activity. The mutant could not accumulate GABA at all the conditions tested except when supplemented with putrescine or spermidine, where high GABA levels were observed in both WT and mutant strains. Glutamate supplementation up to 10 mM for 24 h resulted in a significant increase in both GAD activity and GABA content. Overall results suggested that optimization of nitrogen source and nitrogenous compounds supplementation was effective for the enhancement of GABA accumulation in Synechocystis.





Similar content being viewed by others
References
Alan MK, Frank JT (2000) Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19:479–509
Bouché N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9:110–115
Castanie-Cornet MP, Thomas AP, Dean S, John FE, John WF (1999) Control of acid resistance in Escherichia coli. J Bacteriol 181:3525–3535
Chattopadhyay MK, Tabor H (2013) Polyamines are critical for the induction of the glutamate decarboxylase-dependent acid resistance system in Escherichia coli. J Biol Chem 288:33559–33570
Deng MD, Coleman JR (1999) Ethanol synthesis by genetic engineering in cyanobacteria. Appl Environ Microbiol 65:523–528
Evans PT, Malmberg RL (1989) Do polyamines have roles in plant development? Annu Rev Plant Physiol Plant Mol Biol 40:235–269
Flores E, Herrero A (1994) Assimilatory nitrogen metabolism and its regulation. In: Bryant DA (ed) The molecular biology of cyanobacteria. Kluwer, Dordrecht, pp 487–517
Flores HE, Filner P (1985) Polyamine catabolism in higher plants: characterization of pyrroline dehydrogenase. Plant Growth Regul 3:277–291
Kanwal S, Rastogi RP, Incharoensakdi A (2014) Glutamate decarboxylase activity and gamma-aminobutyric acid content in Synechocystis sp. PCC 6803 under osmotic stress and different carbon sources. J Appl Phycol. doi:10.1007/s10811-014-0259-9
Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff SB, Mickel SJ, McMaster Q, Angst C, Bittiger H, Froestl W, Bettler B (1997) Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature 386:239–246
Khetkorn W, Lindblad P, Incharoensakdi A (2010) Enhanced biohydrogen production by the N2-fixing cyanobacterium Anabaena siamensis strain TISTR 8012. Int J Hydrogen Energy 35:12767–12776
Krnjevic K (1974) Chemical nature of synaptic transmission in vertebrates. Physiol Rev 54:418–540
Labarre J, Thuriaux P, Chauvat F (1987) Genetic analysis of amino acid transport in the facultatively heterotrophic cyanobacterium Synechocystis sp. Strain 6803. J Bacteriol 169:4668–4673
Lindberg P, Park S, Melis A (2010) Engineering a platform for photosynthetic isoprene production in cyanobacteria using Synechocystis as the model organism. Metab Eng 12:70–79
Mann NH (1998) Detecting the environment. In: Whitton BA, Potts M (eds) Ecology of cyanobacteria: their diversity in time and space. Kluwer, Dordrecht, pp 367–395
Meeks JC, Wolk CP, Lockau W, Schilling N, Shaffer PW, Chien WS (1978) Pathways of assimilation of [13N]N2 and 13NH4 + by cyanobacteria with and without heterocysts. J Bacteriol 134:125–130
Schriek S, Rückert C, Staiger D, Pistorius EK, Michel KP (2007) Bioinformatic evaluation of l-arginine catabolic pathways in 24 cyanobacteria and transcriptional analysis of genes encoding enzymes of l-arginine catabolism in the cyanobacterium Synechocystis sp. PCC 6803. BMC Genomics. doi:10.1186/1471-2164-8-437
Scott-Taggart CP, Van Cauwenberghe OR, McLean MD, Shelp BJ (1999) Regulation of γ-aminobutyric acid synthesis in situ by glutamate availability. Physiol Plantarum 106:363–369
Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4:446–452
Sielaff H, Christiansen G, Schwecke T (2006) Natural products from cyanobacteria: exploiting a new source for drug discovery. IDrugs 9:119–127
Steward FC, Durzan DJ (1965) Metabolism of nitrogenous compounds. In: Steward FC (ed) Plant physiology. Academic Press, New York, pp 379–686
Thi T, Binh T, Ju WT, Jung WJ, Park RD (2014) Optimization of γ-amino butyric acid production in a newly isolated Lactobacillus brevis. Biotechnol Lett 36:93–98
Tuin LG, Shelp BJ (1996) In situ [14C] glutamate metabolism by developing soybean cotyledons. II. The importance of glutamate decarboxylation. J Plant Physiol 147:714–720
Wang JJ, Lee CL, Pan TM (2003) Improvement of monacolin K, γ-aminobutyric acid and citrinin production ratio as a function of environmental conditions of Monascus purpureus NTU 601. J Ind Microbiol Biotechnol 30:669–676
Wobeser EAV, Ibelings BW, Bok J, Krasikov V, Huisman J, Matthijs HCP (2011) Concreted changes in gene expression and cell physiology of the cyanobacterium Synechocystis sp. strain PCC 6803 during transitions between nitrogen and light-limited growth. Plant Physiol 155:1445–1457
Wuttinum R, Yodsang P, Maenpaa P, Incharoensakdi A (2009) Characterization of spermidine transport system in a cyanobacterium Synechocystis sp. PCC 6803. J Microbiol Biotechnol 19:447–454
Yang SY, Lu FX, Lu ZX, Bie XM, Jiao Y, Sun LJ, Yu B (2008) Production of gamma-aminobutyric acid by Streptococcus salivarius subsp thermophilus Y2 under submerged fermentation. Amino Acids 34:473–478
Acknowledgments
Simab Kanwal thanks Department of Biochemistry, Faculty of Science and the 90th Anniversary of Chulalongkorn University Ratchadaphiseksomphot Endowment Fund for a PhD scholarship. Wanthanee Khetkorn thanks the Graduate School of Chulalongkorn University for providing postdoctoral fellowship. Aran Incharoensakdi thanks the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University and the Office of Higher Education Commission for the research grants RES560530052-FW and WCU-013-FW-57, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanwal, S., Khetkorn, W. & Incharoensakdi, A. GABA Accumulation in Response to Different Nitrogenous Compounds in Unicellular Cyanobacterium Synechocystis sp. PCC 6803. Curr Microbiol 70, 96–102 (2015). https://doi.org/10.1007/s00284-014-0687-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0687-4