Abstract
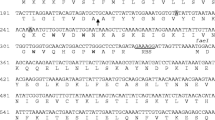
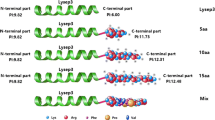
The immunity proteins of pediocin-like bacteriocins possess a positively charged region which is located at the C-terminus in all three subclasses. It has been suggested that this region may be involved in directing the immunity protein to the surface of the bacterial cell membrane. The aim of this study was to determine whether the positively charged residue lysine-46 (K46) around the hydrophobic pocket played a key role for immunity activity of subgroup A immunity protein PedB. At first, heterologous expression of the immune gene pedB from Lactobacillus plantarum BM-1 rendered the sensitive Lactobacillus plantarum WQ0815 resistant to bacteriocin BM-1. Then, using site-directed mutagenesis, the residue K46 was replaced by five different amino-acid residues, including arginine (R), aspartate (D), glutamate (E), glutamine (Q), and threonine (T). Western blot analysis confirmed that all mutated pedB genes were successfully expressed in the host L. plantarum WQ0815. Bacteriocin activity assays subsequently showed that any substitution of the K46 residue significantly reduced its immunity activity. Our present results indicated that the positively charged residue K46 located near the hydrophobic pocket was essential for the functionality of the immunity protein PedB.


Similar content being viewed by others
References
An H, Zhou H, Huang Y, Wang G, Luan C, Mou J, Luo Y, Hao Y (2010) High-level expression of heme-dependent catalase gene katA from Lactobacillus sakei protects Lactobacillus rhamnosus from oxidative stress. Mol Biotechnol 45:155–160
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cintas LM, Rodriguez JM, Fernandez MF, Sletten K, Nes IF, Hernandez PE, Holo H (1995) Isolation and characterization of pediocin L50, a new bacteriocin from Pediococcus acidilactici with a broad inhibitory spectrum. Appl Environ Microbiol 61:2643–2648
Dayem MA, Fleury Y, Devilliers G, Chaboisseau E, Girard R, Nicolas P, Delfour A (1996) The putative immunity protein of the gram-positive bacteria Leuconostoc mesenteroides is preferentially located in the cytoplasm compartment. FEMS Microbiol Lett 138:251–259
De Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Bacteriol 23:130–135
Diep DB, Skaugen M, Salehian Z, Holo H, Nes IF (2007) Common mechanisms of target cell recognition and immunity for class II bacteriocins. P Natl Acad Sci USA 104:2384–2389
Eijsink VGH, Skeie M, Middelhoven PH, Brurberg MB, Nes IF (1998) Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl Environ Microbiol 64:3275–3281
Fimland G, Eijsink VGH, Nissen-Meyer J (2002) Comparative studies of immunity proteins of pediocin-like bacteriocins. Microbiology 148:3661–3670
Holo H, Nilssen O, Nes IF (1991) Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol 173:3879–3887
Jeon HJ, Noda M, Matoba Y, Kumagai T, Sugiyama M (2009) Crystal structure and mutagenic analysis of a bacteriocin immunity protein, Mun-im. Biochem Bioph Res Co 378:574–578
Johnsen L, Dalhus B, Leiros I, Nissen-Meyer J (2005) 1.6-Angstroms crystal structure of EntA-im. A bacterial immunity protein conferring immunity to the antimicrobial activity of the pediocin-like bacteriocin enterocin A. J Biol Chem 280:19045–19050
Johnsen L, Fimland G, Mantzilas D, Nissen-Meyer J (2004) Structure-function analysis of immunity proteins of pediocin-like bacteriocins: C-terminal parts of immunity proteins are involved in specific recognition of cognate bacteriocins. Appl Environ Microbiol 70:2647–2652
Johnsen L, Fimland G, Nissen-Meyer J (2005) The C-terminal domain of pediocin-like antimicrobial peptides (class IIa bacteriocins) is involved in specific recognition of the C-terminal part of cognate immunity proteins and in determining the antimicrobial spectrum. J Biol Chem 280:9243–9250
Kim IK, Kim MK, Kim JH, Yim HS, Cha SS, Kang SO (2007) High resolution crystal structure of PedB, a structural basis for the classification of pediocin-like immunity proteins. BMC Struct Biol 7:35
Kuipers OP, de Ruyter PGGA, Kleerebezem M, deVos WM (1998) Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol 64:15–21
Martin-Visscher LA, Sprules T, Gursky LJ, Vederas JC (2008) Nuclear magnetic resonance solution structure of PisI, a group B immunity protein that provides protection against the type IIa bacteriocin piscicolin 126, PisA. Biochemistry 47:6427–6436
Quadri LE, Kleerebezem M, Kuipers OP, de Vos WM, Roy KL, Vederas JC, Stiles ME (1997) Characterization of a locus from Carnobacterium piscicola LV17B involved in bacteriocin production and immunity: evidence for global inducer-mediated transcriptional regulation. J Bacteriol 179:6163–6171
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Schägger H (2006) Tricine-SDS-PAGE. Nat Protoc 1:16–22
Sørvig E, Grönqvist S, Naterstad K, Mathiesen G, Eijsink VGH, Axelsson L (2003) Construction of vectors for inducible gene expression in Lactobacillus sakei and L. plantarum. FEMS Microbiol Lett 229:119–126
Sprules T, Kawulka KE, Vederas JC (2004) NMR solution structure of ImB2, a protein conferring immunity to antimicrobial activity of the type IIa bacteriocin, carnobacteriocin B2. Biochemistry 43:11740–11749
Thompson K, Collins MA (1996) Improvement in electroporation efficiency for Lactobacillus plantarum by the inclusion of high concentrations of glycine in the growth medium. J Microbiol Meth 26:73–79
van de Guchte M, van der Vossen JM, Kok J, Venema G (1989) Construction of a lactococcal expression vector: expression of hen egg white lysozyme in Lactococcus lactis subsp. lactis. Appl Environ Microbiol 55:224–228
Acknowledgments
We thank Professor Elisabeth Sørvig (Agricultural University of Norway) for kindly providing the plasmid pSIP502. This work was supported by the National Natural Sciences Foundation of China (contract No. 21076223).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Douillard, F.P., Zhou, W. et al. Site-Directed Mutagenesis Identifies the Positively Charged Residue Lysine-46 Essential for the Function of the Immunity Protein PedB. Curr Microbiol 69, 423–428 (2014). https://doi.org/10.1007/s00284-014-0596-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0596-6