Abstract
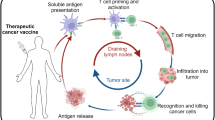
The T-win® technology is an innovative investigational approach designed to activate the body’s endogenous anti-regulatory T cells (anti-Tregs) to target regulatory as well as malignant cells. Anti-Tregs are naturally occurring T cells that can directly react against regulatory immune cells because they recognize proteins that these targets express, including indoleamine 2,3-dioxygenase (IDO), tryptophan 2,6-dioxygenase, arginase, and programmed death ligand 1 (PD-L1). The T-win® technology is characterized by therapeutic vaccination with long peptide epitopes derived from these antigens and therefore offers a novel way to target genetically stable cells with regular human leukocyte antigen expression in the tumor microenvironment. The T-win® technology thus also represents a novel way to attract pro-inflammatory cells to the tumor microenvironment where they can directly affect immune inhibitory pathways, potentially altering tolerance to tumor antigens. The modification of an immune regulatory environment into a pro-inflammatory milieu potentiates effective anti-tumor T cell responses. Many regulatory immune cells may be reverted into effector cells given the right stimulus. Because T-win® technology is based on the immune-modulatory function of the vaccines, the vaccines activate both CD4 and CD8 anti-Tregs. Of importance, in clinical trials, vaccinations against IDO or PD-L1 to potentiate anti-Tregs have so far proved to be safe, with minimal toxicity.

Similar content being viewed by others
References
Scheler M, Wenzel J, Tuting T, Takikawa O, Bieber T, von Bubnoff D (2007) Indoleamine 2,3-dioxygenase (IDO): the antagonist of type I interferon-driven skin inflammation? Am J Pathol 171(6):1936–1943
Popov A, Schultze JL (2008) IDO-expressing regulatory dendritic cells in cancer and chronic infection. J Mol Med 86(2):145–160
Gianchecchi E, Delfino DV, Fierabracci A (2013) Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev 12(11):1091–1100
Andersen MH (2017) Anti-regulatory T cells. Semin Immunopathol 39(3):317–326
Andersen MH (2015) Immune regulation by self-recognition: novel possibilities for anticancer immunotherapy. J Natl Cancer Inst 107(9):154
Yu W, Jiang N, Ebert PJ, Kidd BA, Muller S, Lund PJ et al (2015) Clonal deletion prunes but does not eliminate self-specific alphabeta CD8(+) T lymphocytes. Immunity 42(5):929–941
Sorensen RB, Hadrup SR, Svane IM, Hjortso MC, thor Straten P, Andersen MH (2011) Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood 117(7):2200–2210
Sorensen RB, Berge-Hansen L, Junker N, Hansen CA, Hadrup SR, Schumacher TN et al (2009) The immune system strikes back: cellular immune responses against indoleamine 2,3-dioxygenase. PLoS One 4(9):e6910
Munir S, Andersen GH, Met O, Donia M, Frosig TM, Larsen SK et al (2013) HLA-restricted cytotoxic T cells that are specific for the immune checkpoint ligand PD-L1 occur with high frequency in cancer patients. Cancer Res 73(6):1674–1776
Munir S, Andersen GH, Woetmann A, Odum N, Becker JC, Andersen MH (2013) Cutaneous T cell lymphoma cells are targets for immune checkpoint ligand PD-L1-specific, cytotoxic T cells. Leukemia 27(11):2251–2253
Larsen SK, Munir S, Woetmann A, Froesig TM, Odum N, Svane IM et al (2013) Functional characterization of Foxp3-specific spontaneous immune responses. Leukemia 27(12):2332–2340
Martinenaite E, Ahmad SM, Hansen M, Met O, Westergaard MW, Larsen SK et al (2016) CCL22-specific T cells: modulating the immunosuppressive tumor microenvironment. Oncoimmunology 5(11):e1238541
Martinenaite E, Mortensen RE, Hansen M, Holmstrom MO, Ahmad SM, Met O et al (2017) Frequent spontaneous adaptive immune responses towards arginase. Oncoimmunology 7:e1404215. https://doi.org/10.1080/2162402X.2017.1404215
Hjortso MC, Larsen SK, Kongsted P, Met O, Frosig TM, Andersen GH et al (2015) Tryptophan 2,3-dioxygenase (TDO)-reactive T cells differ in their functional characteristics in health and cancer. Oncoimmunology 4(1):e968480
Ahmad SM, Martinenaite E, Holmstrom MO, Jorgensen M, Met O, Nastasi C et al (2017) The inhibitory checkpoint, PD-L2, is a target for effector T cells: novel possibilities for immune therapy. Oncoimmunolgy. https://doi.org/10.1080/2162402X.2017.1390641
Andersen MH (2018) The balance players of the adaptive immune system. Cancer Res 78(6):1379–1382
Gulley JL, Madan RA, Pachynski R, Mulders P, Sheikh NA, Trager J et al (2017) Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J Natl Cancer Inst 109(4):2982600
Prendergast GC, Metz R, Muller AJ (2009) IDO recruits Tregs in melanoma. Cell Cycle 8(12):1818–1819
Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M, Waeltz P, Bowman KJ, Polam P, Sparks RB, Yue EW, Li Y, Wynn R, Fridman JS, Burn TC, Combs AP, Newton RC, Scherle PA (2010) Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 115(17):3520–3530
Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE et al (2012) IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov 2(8):722–735
Brochez L, Chevolet I, Kruse V (2017) The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer 76:167–182. https://doi.org/10.1016/j.ejca.2017.01.011
Mullard A (2018) IDO takes a blow. Nat Rev Drug Discov 17(5):307
Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J et al (2014) Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from Indoleamine 2,3 dioxygenase. Clin Cancer Res 20(1):221–232
Nagata Y, Hanagiri T, Mizukami M, Kuroda K, Shigematsu Y, Baba T, Ichiki Y, Yasuda M, So T, Takenoyama M, Sugio K, Nagashima A, Yasumoto K (2009) Clinical significance of HLA class I alleles on postoperative prognosis of lung cancer patients in Japan. Lung Cancer 65(1):91–97
Bjoern J, Iversen TZ, Nitschke NJ, Andersen MH, Svane IM (2016) Safety, immune and clinical responses in metastatic melanoma patients vaccinated with a long peptide derived from indoleamine 2,3-dioxygenase in combination with ipilimumab. Cytotherapy 18(8):1043–1055
Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R, de Plaen E, Uyttenhove C, Wouters J, Masereel B, van den Eynde BJ (2012) Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci U S A 109(7):2497–2502
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478(7368):197–203
Yu CP, Song YL, Zhu ZM, Huang B, Xiao YQ, Luo DY (2017) Targeting TDO in cancer immunotherapy. Med Oncol 34(5):73–0933
Hsu YL, Hung JY, Chiang SY, Jian SF, Wu CY, Lin YS, Tsai YM, Chou SH, Tsai MJ, Kuo PL (2016) Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget 7(19):27584–27598
Munir S, Larsen SK, Iversen TZ, Donia M, Klausen TW, Svane IM, Straten P, Andersen MH (2012) Natural CD4(+) T-cell responses against Indoleamine 2,3-dioxygenase. PLoS One 7(4):e34568
Chen Z, O'Shea JJ (2008) Th17 cells: a new fate for differentiating helper T cells. Immunol Res 41(2):87–102
Zou W, Restifo NPT (2010) (H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol 10(4):248–256
Sakaguchi S (2006) Regulatory T cells. Springer Semin Immunopathol 28(1):1–2
Sundrud MS, Trivigno C (2013) Identity crisis of Th17 cells: many forms, many functions, many questions. Semin Immunol 25(4):263–272
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel III EE, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T (2018) TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554(7693):544–548
Gajewski TF, Schreiber H, Fu YX (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14(10):1014–1022
Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM et al (2009) Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 5(4):e1000371
Kozako T, Yoshimitsu M, Fujiwara H, Masamoto I, Horai S, White Y, Akimoto M, Suzuki S, Matsushita K, Uozumi K, Tei C, Arima N (2009) PD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patients. Leukemia 23(2):375–382
Atanackovic D, Luetkens T, Kroger N (2013) Coinhibitory molecule PD-1 as a potential target for the immunotherapy of multiple myeloma. Leukemia 28:993–1000. https://doi.org/10.1038/leu.2013.310
Yang H, Bueso-Ramos C, Dinardo C, Estecio MR, Davanlou M, Geng QR et al (2014) Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 28(6):1280–1288
Krejsgaard T, Odum N, Geisler C, Wasik MA, Woetmann A (2012) Regulatory T cells and immunodeficiency in mycosis fungoides and Sezary syndrome. Leukemia 26(3):424–432
Kollgaard T, Petersen SL, Hadrup SR, Masmas TN, Seremet T, Andersen MH, Madsen HO, Vindeløv L, thor Straten P (2005) Evidence for involvement of clonally expanded CD8+ T cells in anticancer immune responses in CLL patients following nonmyeloablative conditioning and hematopoietic cell transplantation. Leukemia 19(12):2273–2280
Ame-Thomas P, Le PJ, Yssel H, Caron G, Pangault C, Jean R et al (2012) Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia 26(5):1053–1063
van de Donk NW, Kamps S, Mutis T, Lokhorst HM (2012) Monoclonal antibody-based therapy as a new treatment strategy in multiple myeloma. Leukemia 26(2):199–213
Tamura H, Ishibashi M, Yamashita T, Tanosaki S, Okuyama N, Kondo A, Hyodo H, Shinya E, Takahashi H, Dong H, Tamada K, Chen L, Dan K, Ogata K (2013) Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia 27(2):464–472
Greaves P, Gribben JG (2013) The role of B7 family molecules in hematologic malignancy. Blood 121(5):734–744
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P et al (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366(26):2455–2465
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2453
Munir S, Andersen GH, Svane IM, Andersen MH (2013) The immune checkpoint regulator PD-L1 is a specific target for naturally occurring CD4+ T cells. Oncoimmunology 2(4):e23991
Ahmad SM, Larsen SK, Svane IM, Andersen MH (2014) Harnessing PD-L1-specific cytotoxic T cells for anti-leukemia immunotherapy to defeat mechanisms of immune escape mediated by the PD-1 pathway. Leukemia 28(1):236–238
Minami T, Minami T, Shimizu N, Yamamoto Y, De VM, Nozawa M et al (2015) Identification of programmed death ligand 1-derived peptides capable of inducing cancer-reactive cytotoxic T lymphocytes from HLA-A24+ patients with renal cell carcinoma. J Immunother 38(7):285–291
Dong H, Strome SE, Matteson EL, Moder KG, Flies DB, Zhu G, Tamura H, Driscoll CLW, Chen L (2003) Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest 111(3):363–370
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10(9):942–949
Ishida T, Ishii T, Inagaki A, Yano H, Komatsu H, Iida S, Inagaki H, Ueda R (2006) Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res 66(11):5716–5722
Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, Perez S, Pasqual N, Faure C, Ray-Coquard I, Puisieux A, Caux C, Blay JY, Menetrier-Caux C (2009) Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res 69(5):2000–2009
Cao L, Hu X, Zhang J, Huang G, Zhang Y (2014) The role of the CCL22-CCR4 axis in the metastasis of gastric cancer cells into omental milky spots. J Transl Med 12:267. https://doi.org/10.1186/s12967-014-0267-1.:267-0267.
Ahmad SM, Martinenaite E, Hansen M, Junker N, Borch TH, Met O et al (2016) PD-L1 peptide co-stimulation increases immunogenicity of a dendritic cell-based cancer vaccine. Oncoimmunology 5(8):e1202391
Sources of support
This work was supported by Herlev Hospital, the Danish Cancer Society, and the Danish Council for Independent Research. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MHA is an author of several filed patent applications based on the use of CCL2, CCL22, PD-L1, PD-L2, arginase, TDO, or IDO for vaccination. The rights of the patent applications have been transferred to Copenhagen University Hospital, Herlev, according to the Danish Law of Public Inventions at Public Research Institutions. The capital region has licensed some of these patents to the company IO Biotech ApS. MHA is a shareholder and board member of the IO Biotech ApS, which has the purpose of developing immune-modulating vaccines for cancer treatment.
Additional information
This article is a contribution to the special issue on Anti-cancer Immunotherapy: Breakthroughs and Future Strategies - Guest Editor: Mads Hald Andersen
Rights and permissions
About this article
Cite this article
Andersen, M.H. The T-win® technology: immune-modulating vaccines. Semin Immunopathol 41, 87–95 (2019). https://doi.org/10.1007/s00281-018-0695-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-018-0695-8