Abstract
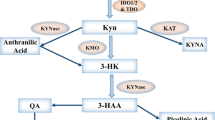
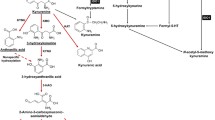
Tryptophan-2,3-dioxygenase (TDO) is a homotetrameric heme-containing protein catalyzing the initial step in the kynurenine pathway, which oxidates the 2,3-double bond of the indole ring in l-tryptophan and catalyzes it into kynurenine (KYN). The upregulation of TDO results in a decrease in tryptophan and the accumulation of KYN and its metabolites. These metabolites can affect the proliferation of T cells. Increasing evidence demonstrates that TDO is a promising therapeutic target in the anti-tumor process. Despite its growing popularity, there are only a few reviews focusing on TDO in tumors. Hence, we herein review the biological features and regulatory mechanisms of TDO. Additionally, we focus on the role of TDO in the anti-tumor immune response in different tumors. Finally, we also provide our viewpoint regarding the future developmental directions of TDO in cancer research, especially in relation to the development and application of TDO inhibitors as novel cancer treatments.



Similar content being viewed by others
References
Phillips RS. Structure, mechanism, and substrate specificity of kynureninase. J Biochim Biophys Acta. 2011;1814(1814):1481–8. doi:10.1016/j.bbapap.2010.12.003.
Hayaishi O. Utilization of superoxide anion by indoleamine oxygenase-catalyzed tryptophan and indoleamine oxidation. Recent Adv Tryptophan Res. 1996;. doi:10.1007/978-1-4613-0381-7_45.
Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. J Immunology. 2002;107(4):452–60. doi:10.1046/j.1365-2567.2002.01526.x.
Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002;101(2):151–5. doi:10.1002/ijc.10645.
Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–8. doi:10.4049/jimmunol.0903670.
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. J Nat. 2011;478(7368):197–203. doi:10.1038/nature10491.
Kotake Y, Masayama IZ. Uber den Mechanismus der kynureninbildung aus tryptophan. J Z Physiol Chem. 1936;243:237–44.
Hu X, Bao Z, Hu J, Shao M, Zhang L, Bi K, Zhan A, Huang X. Cloning and characterization of tryptophan-2,3-dioxygenase gene of Zhikong scallop Chlamys farreri (Jones and Preston 1904). J Aquac Res. 2006;37:1187–94.
Mukabayire O, Cornel AJ, Dotson EM, Collins FH, Besansky NJ. The tryptophan oxygenase gene of Anopheles gambiae. J Insect Biochem Mol Biol. 1996;26:525–8. doi:10.1016/S0965-1748(96)00026-4.
Lorenzen MD, Brown SJ, Denell RE, Beeman RW. Cloning and characterization of the Tribolium castaneum eye-color genes encoding tryptophan oxygenase and kynurenine 3-monoxygenase. J Genet. 2002;160:225–34.
Fabrick JA, Kanost MR, Baker JE. RNAi-induced silencing of embryonic tryptophan oxygenase in the Pyralid moth, Plodia interpunctella. J Insect Sci. 2004;4(1):103–8. doi:10.1673/031.004.1501.
Searles LL, Voelker RA. Molecular characterization of the drosophila vermilion locus and its suppressible alleles. J Proc Natl Acad Sci USA. 1986;83:404–8.
Tshimura Y, Nozaki M, Hayaishi O. The oxygenated form of l-tryptophan 2,3-dioxygenase as reaction intermediate. J Biol Chem. 1970;245(14):3593–602.
Iwamoto Y, Lee IS, Tsubaki M, Kido R. Tryptophan-2, 3-dioxygenase in Saccharomyces cerevisiae. Can J Microbiol. 1995;141:19–26.
Matsumura M, Osada K, Aiba S. L-tryptophan-2, 3-dioxygenase of a moderate thermophile, Bacillus brevis, purification, properties and a substrate-mediated stabilization of the quaternary structure. Biochim Biophys Acta. 1984;786:9–17.
Ball HJ, Jusof FF, Bakmiwewa SM, Hunt NH, Yuasa HJ. Tryptophan-catabolizing enzymes-party of three. J Front Immunol. 2014;5(485):1–10. doi:10.3389/fimmu.2014.00485.
Comings DE, Muhleman D, Dietz G, Sherman M, Forest GL. Sequence of human tryptophan 2, 3-dioxygenase (TDO2): presence of a glucocorticoid response-like element composed of a GTT repeat and an intronic CCCCT repeat. J Genomics. 1995;29(2):390–6. doi:10.1006/geno.1995.9990.
Gongkai JIAO, Xiao-yan KE, Lu CHENG, Ying ZHOU, Ping CHEN, Bei-li SUN, et al. Positive association between tryptophan-2, 3-dioxygenase gene polymorphism and autistic disorder in Chinese Han population. Chin Med J. 2010;123(2):82.
Comings DE, Gade R, Muhleman D, Chiu C, Wu S, To M, et al. Exon and intron variants in the human tryptophan 2, 3 dioxygenase gene: potential association with Tourette syndrome, substance abuse and other disorders. J Pharmacogenet Genomics. 1996;6(4):307–18. doi:10.1097/00008571-199608000-00004.
Britan A, Maffre V, Tone S, Vet JR. Quantitative and spatial differences in the expression of tryptophan metabolizing enzymes in mouse epididymis. J Cell Tissue Res. 2006;324(2):301–10. doi:10.1007/s00441-005-0151-7.
Minatogawa Y, Suzuki S, Ando Y, Tone S, Takikawa O. Tryptophan pyrrole ring cleavage enzymes in placenta. J Adv Exp Med Biol. 2003;527:425–34. doi:10.1007/978-1-4615-0135-0_50.
Haber R, Bessette D, Hulihan-Giblin B, Durcan MJ, Goldman D. Identification of tryptophan-2, 3-dioxygenase RNA in rodent brain. J Neurochem. 1993;60:1159–62.
Doherty LF, Kwon HE, Taylor HS. Regulation of tryptophan 2,3-dioxygenase by HOXA10 enhances embryo viability through serotonin signaling.J. Am J Physiol Endocrinol Metab. 2011;300(1):86–93. doi:10.1152/ajpendo.00439.2010.
Wu W, Nicolazzo JA, Wen L, Chung R, Stankovic R, Bao SS, et al. Expression of tryptophan-2, 3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS One. 2013;8:e59749.
Miller C, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S. Expression of the kynurenine pathway enzyme tryptophan 2, 3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. J Neurobiol Dis. 2004;15(3):618–29. doi:10.1016/j.nbd.2003.12.015.
Yu CP, Pan ZZ, Luo DY. TDO as a therapeutic target in brain diseases J. Metab Brain Dis. 2016;31(4):737–47. doi:10.1007/s11011-016-9824-z.
Mørland J, Stowell L, Gjerde H. Ethanol increases rat liver tryptophan oxygenase: evidence for corticosterone mediation. Alcohol. 1985;2:255–9. doi:10.1016/0741-8329(85)90055-2.
Badawy AA. Tryptophan metabolism in alcoholism. Nutr Res Rev. 2002;15:123–52. doi:10.1079/NRR200133.
Mehler AH, Knox WE. The conversion of tryptophan to Kynurenine in liver II. The enzymatic hydrolysis of formylkynurenine. J Biol Chem. 1950;187(1):431–8.
Knox WE, Mehler AH. The adaptive increase of the tryptophan peroxidase-oxidase system of liver. J Sci. 1951;113(2931):237–8.
Danesch U, Gloss B, Schmid W, Schütz G, Schüle R, Renkawitz R. Glucocorticoid induction of the rat tryptophan oxygenase gene is mediated by two widely separated glucocorticoid-responsive elements. Embo J. 1987;6(3):625–30.
Ren S, Correia MA. Heme: a regulator of rat hepatic tryptophan 2,3-dioxygenase? J Arch Biochem Biophys. 2000;377(1):195–203. doi:10.1006/abbi.2000.1755.
Nakamura T, Shinno HI, Chihara A. Insulin and glucagon as a new regulator system for tryptophan oxygenase activity demonstrated in primary cultured rat hepatocytes. J Biol Chem. 1980;255:7533–5.
Braidman IP, Rose DP. Effects of sex hormones on three glucocorticoid inducible enzymes concerned with amino acid metabolism in rat liver. J Endocrinol. 1971;89:1250–5.
Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frédérick R, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. J Proc Natl Acad Sci USA. 1971;109(7):2497–502.
D’Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, et al. TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. J Cancer Res. 2015;75(21):4651–64. doi:10.1158/0008-5472.CAN-15-2011.
Hsu Ya-Ling, Hung Jen-Yu, Chiang Shin-Yi, Jian Shu-Fang, Cheng-Ying Wu, Lin Yi-Shiuan, et al. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. J Oncotarget. 2014;7(19):27584–98. doi:10.18632/oncotarget.8488.
Chen IC, Lee KH, Hsu YH, et al. Expression pattern and clinicopathological relevance of the indoleamine 2,3-dioxygenase 1/tryptophan 2,3-dioxygenase protein in colorectal cancer. J Dis Markers. 2016;2016(3):1–9. doi:10.1155/2016/8169724.
Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C. Tryptophan catabolism generates autoimmune- preventive regulatory T cells. Transp Immunol. 2006;17(1):58–60. doi:10.1016/j.trim.2006.09.017.
Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–42. doi:10.1016/j.immuni.2005.03.013.
Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. J. Nat Rev Immunol. 2007;7(10):817–23. doi:10.1038/nri2163.
Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Taylor PA, Mellor AL, Munn DH, et al. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood. 2008;111(6):3257–65. doi:10.1182/blood-2007-06-096081.
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi:10.1038/nature10491.
Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787. doi:10.1182/blood-2007-06-09608.
Hayashi T, Raz E. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci. 2007;104(47):18619–24. doi:10.1073/pnas.0709261104.
Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–61. doi:10.4049/jimmunol.176.11.6752.
Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069–77. doi:10.1038/sj.cdd.4401073.
Weber WP, Feder-Mengus C, Chiarugi A, Rosenthal R, Reschner A, Schumacher R, et al. Differential effects of the tryptophan metabolite 3-hydroxyanthranilic acid on the proliferation of human CD8+ T cells induced by TCR triggering or homeostatic cytokines. Eur J Immunol. 2006;36(2):296–304. doi:10.1002/eji.200535616.
Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. J Sci Transl Med. 2010;2(32):32ra36. doi:10.1126/scitranslmed.3000632.
Zaher SS, Germain C, Fu H, Larkin DF, George AJ. 3-hydroxykynurenine suppresses CD4+ T-cell proliferation, induces T-regulatory-cell development, and prolongs corneal allograft survival. Invest Ophthalmol Vis Sci. 2011;52(5):2640–8. doi:10.1167/iovs.10-5793.
Mi YK, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19(17):1951. doi:10.1101/gad.1331805.
Sahm F, Oezen I, Opitz CA, Radlwimmer B, von Deimling A, Ahrendt T, et al. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. J Cancer Res. 2013;73(11):3225–34. doi:10.1158/0008-5472.
Di Serio C, Cozzi A, Angeli I, Doria L, Micucci I, Pellerito S, et al. Kynurenic acid inhibits the release of the neurotrophic fibroblast growth factor (FGF)-1 and enhances proliferation of glia cells, in vitro. Cell Mol Neurobiol. 2005;25(6):981–93. doi:10.1007/s10571-005-8469-y.
Bresjanac M, Antauer G. Reactive astrocytes of the quinolinic acid-lesioned rat striatum express GFRalpha1 as well as GDNF in vivo. J Exp Neurol. 2000;164(1):53–9. doi:10.1006/exnr.2000.7416.
Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC, et al. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11(3):312–9. doi:10.1038/nm1196.
Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–54. doi:10.1172/JCI31178.
Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–74. doi:10.1038/nm934.
Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396(1):203–13.
Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67(67):7082–7. doi:10.1158/0008-5472.
Löb S, Königsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: Can we see the wood for the trees? Nat Rev Cancer. 2009;9(6):445. doi:10.1038/nrc2639.
Soliman H, Rawal B, Fulp J, Lee JH, Lopez A, Bui MM, et al. Analysis of indoleamine 2-3 dioxygenase (IDO1) expression in breast cancer tissue by immunohistochemistry. Cancer Immunol Immunother. 2013;62(5):829–37. doi:10.1007/s00262-013-1393-y.
Astigiano S, Morandi B, Costa R, Mastracci L, D’Agostino A, Ratto GB, Melioli G, Frumento G. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. J Neoplasia. 2005;7(4):390–6. doi:10.1593/neo.04658.
Schmidt SK, Siepmann S, Kuhlmann K, Meyer HE, Metzger S, Pudelko S, et al. Influence of tryptophan contained in 1-methyl-tryptophan on antimicrobial and immunoregulatory functions of indoleamine 2,3-dioxygenase. Plos One. 2012;7(7):e44797. doi:10.1371/journal.Pone.0044797.
Wenzel H. The role of indoleamine 2,3-dioxygenase (IDO) in immune tolerance: focus on macrophage polarization of THP-1 cells. Cell Immunol. 2014;289(1–2):42–8. doi:10.1016/j.Cellimm.2014.02.005.
Frumento Guido, Rotondo Rita, Tonetti Michela, Damonte Gianluca, Benatti Umberto, Ferrara Giovanni Battista. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196(4):459–68. doi:10.1084/jem.20020121.
Song H, Park H, Kim YS, Kim KD, Lee HK, Cho DH, et al. L-kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species. Int Immunopharmacol. 2011;11(8):932–8. doi:10.1016/j.intimp.2011.02.005.
Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. 2015;99:180–5. doi:10.1016/j.addr.2015.11.009.
Gonzalezgugel E, Saxena M, Bhardwaj N. Modulation of innate immunity in the tumor microenvironment.J. Cancer Immunol Immunother. 2016;65(10):1–8. doi:10.1007/s00262-016-1859-9.
Burga Rachel A, Nguyen Tuongvan, Zulovich Jane, Madonna Sarah, Ylisastigui Loyda, Fernandes Rohan. Improving efficacy of cancer immunotherapy by genetic modification of natural killer cells J. Cytotherapy. 2016;18(11):1410–21. doi:10.1016/j.jcyt.2016.05.018.
Shissler SC, Bollino DR, Tiper IV, Bates JP, Derakhshandeh R, Webb TJ. Immunotherapeutic strategies targeting natural killer T cell responses in cancer J. Immunogenetics. 2016;8:1–16. doi:10.1007/s00251-016-0928-8.
Salter M, Hazelwood R, Pogson CI, Iyer R, Madge DJ. The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism in the rat. J Biochem Pharmacol. 1995;49(10):1435–42. doi:10.1016/0006-2952(95)00006-L.
Gibney SM, Fagan EM, Waldron AM, O’Byrne J, Connor TJ, Harkin A. Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int J Neuropsychopharmacol. 2014;17(6):917–28. doi:10.1017/S1461145713001673.
Wu JS, Lin SY, Liao FY, Hsiao WC, Lee LC, Peng YH, et al. Identification of substituted naphthotriazolediones as novel tryptophan 2,3-dioxygenase (TDO) inhibitors through structure-based virtual screening. J Med Chem. 2015;58(19):7807–19. doi:10.1021/acs.jmedchem.5b00921.
Abdelmagid AF. Targeting the inhibition of tryptophan 2,3-dioxygenase (TDO-2) for cancer treatment. ACS Med Chem Lett. 2017;8:11–3. doi:10.1021/acsmedchemlett.6b00458.
Acknowledgments
We thank the participants in this study. This work was supported by grants from National Natural Science Foundation of China (Grant Nos. 81160248 and 81560464 to DYL, and Grant No. 81560389 to ZMZ), Natural Science Foundation of Jiangxi Province (Grant No. 20151BAB205058 to DYL) and Innovation Foundation for Graduate Students of Nanchang University (Grant No. CX2015181 to YLS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Yu, CP., Song, YL., Zhu, ZM. et al. Targeting TDO in cancer immunotherapy. Med Oncol 34, 73 (2017). https://doi.org/10.1007/s12032-017-0933-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-0933-2