Abstract
Purpose
Midostaurin, approved for treating FLT-3-mutated acute myeloid leukemia and advanced systemic mastocytosis, is metabolized by cytochrome P450 (CYP) 3A4 to two major metabolites, and may inhibit and/or induce CYP3A, CYP2B6, and CYP2C8. Two studies investigated the impact of midostaurin on CYP substrate drugs and oral contraceptives in healthy participants.
Methods
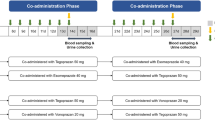
Using sentinel dosing for participants’ safety, the effects of midostaurin at steady state following 25-day (Study 1) or 24-day (Study 2) dosing with 50 mg twice daily were evaluated on CYP substrates, midazolam (CYP3A4), bupropion (CYP2B6), and pioglitazone (CYP2C8) in Study 1; and monophasic oral contraceptives (containing ethinylestradiol [EES] and levonorgestrel [LVG]) in Study 2.
Results
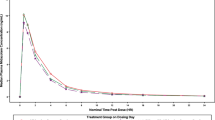
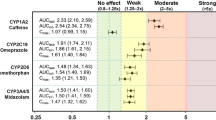
In Study 1, midostaurin resulted in a 10% increase in midazolam peak plasma concentrations (Cmax), and 3–4% decrease in total exposures (AUC). Bupropion showed a 55% decrease in Cmax and 48–49% decrease in AUCs. Pioglitazone showed a 10% decrease in Cmax and 6% decrease in AUC. In Study 2, midostaurin resulted in a 26% increase in Cmax and 7–10% increase in AUC of EES; and a 19% increase in Cmax and 29–42% increase in AUC of LVG. Midostaurin 50 mg twice daily for 28 days ensured that steady-state concentrations of midostaurin and the active metabolites were achieved by the time of CYP substrate drugs or oral contraceptive dosing. No safety concerns were reported.
Conclusion
Midostaurin neither inhibits nor induces CYP3A4 and CYP2C8, and weakly induces CYP2B6. Midostaurin at steady state has no clinically relevant PK interaction on hormonal contraceptives. All treatments were well tolerated.



Similar content being viewed by others
Availability of data and materials
Novartis is committed to sharing with qualified external researchers, access to patient/participant-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients/participants who have participated in the trial in line with applicable laws and regulations.
Change history
05 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00280-024-04657-5
References
EMA (2017) Rydapt. https://www.ema.europa.eu/en/medicines/human/EPAR/rydapt. Accessed 25 Apr 2023
FDA (2021) RYDAPT® (midostaurin) capsules, for oral use Initial US. Approval: 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/207997s008lbledt.pdf. Accessed 09 Sep 2023
Gu H, Dutreix C, Rebello S, Ouatas T, Wang L, Chun DY, Einolf HJ, He H (2018) Simultaneous physiologically based pharmacokinetic (PBPK) modeling of parent and active metabolites to investigate complex CYP3A4 drug-drug interaction potential: a case example of midostaurin. Drug Metab Dispos 46(2):109–121. https://doi.org/10.1124/dmd.117.078006
He H, Tran P, Gu H, Tedesco V, Zhang J, Lin W, Gatlik E, Klein K, Heimbach T (2017) Midostaurin, a novel protein kinase inhibitor for the treatment of acute myelogenous leukemia: insights from human absorption, metabolism, and excretion studies of a BDDCS II drug. Drug Metab Dispos 45(5):540–555. https://doi.org/10.1124/dmd.116.072744
Nordt SP, Clark RF (1997) Midazolam: a review of therapeutic uses and toxicity. J Emerg Med 15(3):357–365. https://doi.org/10.1016/S0736-4679(97)00022-X
FDA (2017) WELLBUTRIN® (bupropion hydrochloride) tablets, for oral use Initial US. Approval: 1985. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
FDA (2011) ACTOS (pioglitazone hydrochloride) tablets for oral use Initial US. Approval: 1999 https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021073s043s044lbl.pdf. Accessed 5 May 2023
FDA (2020) In vitro drug interaction studies—cytochrome P450 enzyme- and transporter-mediated drug interactions guidance for industry. https://www.fda.gov/media/134582/download. Accessed 18 Jun 2023
EMA (2012) EMA Guideline on the investigation of drug interactions. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions-revision-1_en.pdf. Accessed 18 Jun 2023
Gibbons JA, de Vries M, Krauwinkel W, Ohtsu Y, Noukens J, van der Walt JS, Mol R, Mordenti J, Ouatas T (2015) Pharmacokinetic drug interaction studies with enzalutamide. Clin Pharmacokinet 54(10):1057–1069. https://doi.org/10.1007/s40262-015-0283-1
Bosilkovska M, Samer CF, Déglon J, Rebsamen M, Staub C, Dayer P, Walder B, Desmeules JA, Daali Y (2014) Geneva cocktail for cytochrome P450 and P-glycoprotein activity assessment using dried blood spots. Clin Pharmacol Ther 96(3):349–359. https://doi.org/10.1038/clpt.2014.83
Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y (2013) Investigation into CYP3A4-mediated drug–drug interactions on midostaurin in healthy volunteers. Cancer Chemother Pharmacol 72(6):1223–1234. https://doi.org/10.1007/s00280-013-2287-6
FDA (2020) Clinical drug interaction studies—cytochrome P450 enzyme- and transporter-mediated drug interactions guidance for industry. https://www.fda.gov/media/134581/download. Accessed 23 Jun 2023
Sechaud R, Sinclair K, Grosch K, Ouatas T, Pathak D (2022) Evaluation of drug-drug interactions between midostaurin and strong CYP3A4 inhibitors in patients with FLT-3-mutated acute myeloid leukemia (AML). Cancer Chemother Pharmacol 90(1):19–27. https://doi.org/10.1007/s00280-022-04448-w
Gu H, Sechaud R, Hanna I, Pelis R, Einolf H (manuscript in preparation) Physiologically based pharmacokinetic modeling of midostaurin and metabolites at steady state to bridge drug interaction scenarios in lieu of clinical trials
Acknowledgements
The authors thank all the investigators, trial site staff, and volunteers who participated in the trials. The authors thank Vennila Dharman MBBS (Novartis Healthcare Pvt. Ltd. India) for providing medical writing support in accordance with Good Publication Practice (GPP4) guidelines.
Funding
The studies were funded by Novartis.
Author information
Authors and Affiliations
Contributions
RS, HG, GR, AT, OC, GKS, AB, and HDM performed conception and design, data review and interpretation, drafting the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
R Sechaud, G Rahmanzadeh, and H Menssen are employees of Novartis Pharma AG, Switzerland. H Gu, A Taylor, O Chiparus, are employees of Novartis Pharmaceuticals Corporation, USA. GK Sharma is an employee of Novartis Healthcare Pvt. Ltd., India. A Breitschaft is an employee of Parexel International GmbH, Germany.
Ethical approval and informed consent
Both the studies were performed in accordance with the ethical principles, which have their origin in the Declaration of Helsinki and are consistent with Good Clinical Practice and applicable regulatory requirements. All participants of the study provided written informed consent according to international standards. Both studies were conducted at the Early Phase Clinical Unit of PAREXEL International GmbH in Berlin, Germany. The study protocols were reviewed and approved by the State Office of Health and Social Affairs Ethics Committee of Berlin (Landesamt für Gesundheitund Soziales Ethik-Kommission des Landes Berlin) for PAREXEL International GmbH. Study 1 with CYP substrates and Study 2 with oral contraceptives were registered under EudraCT number: 2018-002786-19 and EudraCT number: 2018-002867-25, respectively, and both obtained a favorable opinion from the IEC. The study participants were informed about the study procedures, risks, and benefits of their participation. Informed consent was documented by the investigator.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to a retrospective open access cancellation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sechaud, R., Gu, H., Rahmanzadeh, G. et al. Midostaurin drug interaction profile: a comprehensive assessment of CYP3A, CYP2B6, and CYP2C8 drug substrates, and oral contraceptives in healthy participants. Cancer Chemother Pharmacol 93, 439–453 (2024). https://doi.org/10.1007/s00280-023-04635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04635-3