Abstract
Purpose
This study aims to clarify the impact of CYP3A5 and ABCB1 polymorphisms on the pharmacokinetics of vincristine (VCR) in adult patients receiving CHOP therapy.
Methods
Plasma samples were collected immediately after the end of VCR administration and at 1.5, 2.5, 3.5, 5.5, 9.5, 13.5, and 25.5 h after the start of administration. Areas under the plasma concentration–time curves of VCR in the elimination phase (AUC1.5–25.5) were calculated using the linear trapezoidal rule. Half-lives of VCR during the early phase (1.5–5.5 h) and terminal phase (5.5–25.5 h; t1/2γ) were determined according to the log-linear regression of the concentration–time data for at least 3 sampling points.
Results
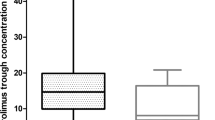
A total of 41 adult patients were enrolled in this study. The median t1/2γ and AUC1.5–25.5 were significantly longer and higher in CYP3A5 non-expressers (CYP3A5*3/*3) than in CYP3A5 expressers (CYP3A5*1/*1 or *1/*3) (21.3 vs 13.8 h, P = 0.005 and 35.5 vs 30.0 ng・h/mL, P = 0.006, respectively). Conversely, there were no significant differences in pharmacokinetic parameters among the ABCB1 c.1236C>T, c.2677G>A/T, c.3435C>T genotype groups. A stepwise selection multiple linear regression analysis showed that the dose of VCR administered and CYP3A5 non-expresser status were independent factors influencing the AUC1.5–25.5 (partial R2 = 0.212, P = 0.002 and partial R2 = 0.143, P = 0.010, respectively).
Conclusion
The CYP3A5*3 polymorphism was found to be an indicator for predicting exposure to VCR in adult patients receiving CHOP therapy. This information may be useful for the individualization of VCR dosages.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Carozzi VA, Canta A, Chiorazzi A (2015) Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? Neurosci Lett 596:90–107. https://doi.org/10.1016/j.neulet.2014.10.014
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4(4):253–265. https://doi.org/10.1038/nrc1317
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R et al (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346(4):235–242. https://doi.org/10.1056/NEJMoa011795
Haim N, Epelbaum R, Ben-Shahar M, Yarnitsky D, Simri W, Robinson E (1994) Full dose vincristine (without 2-mg dose limit) in the treatment of lymphomas. Cancer 73(10):2515–2519. https://doi.org/10.1002/1097-0142(19940515)73:10%3c2515::aid-cncr2820731011%3e3.0.co;2-g
Sethi VS, Jackson DV Jr, White DR, Richards F 2nd, Stuart JJ, Muss HB et al (1981) Pharmacokinetics of vincristine sulfate in adult cancer patients. Cancer Res 41(9 Pt 1):3551–3555
Igarashi T, Kishi S, Hosono N, Higashi T, Iwao T, Yano R et al (2021) Population pharmacokinetic model development and exposure-response analysis of vincristine in patients with malignant lymphoma. Cancer Chemother Pharmacol 87(4):501–511. https://doi.org/10.1007/s00280-020-04220-y
Wu CY, Li GT, Chu CC, Guo HL, Fang WR, Li T et al (2023) Proactive therapeutic drug monitoring of vincristine in pediatric and adult cancer patients: current supporting evidence and future efforts. Arch Toxicol 97(2):377–392. https://doi.org/10.1007/s00204-022-03418-8
Dennison JB, Renbarger JL, Walterhouse DO, Jones DR, Hall SD (2008) Quantification of vincristine and its major metabolite in human plasma by high-performance liquid chromatography/tandem mass spectrometry. Ther Drug Monit 30(3):357–364. https://doi.org/10.1097/FTD.0b013e31816b92c9
Song S, Suzuki H, Kawai R, Sugiyama Y (1999) Effect of PSC 833, a P-glycoprotein modulator, on the disposition of vincristine and digoxin in rats. Drug Metab Dispos 27(6):689–694
Ahmed S, Zhou Z, Zhou J, Chen SQ (2016) Pharmacogenomics of drug metabolizing enzymes and transporters: relevance to precision medicine. Genom Proteom Bioinform 14(5):298–313. https://doi.org/10.1016/j.gpb.2016.03.008
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J et al (2001) Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Gene 27(4):383–391. https://doi.org/10.1038/86882
Skiles JL, Chiang C, Li CH, Martin S, Smith EL, Olbara G et al (2018) CYP3A5 genotype and its impact on vincristine pharmacokinetics and development of neuropathy in Kenyan children with cancer. Pediatr Blood Cancer. https://doi.org/10.1002/pbc.26854
Guilhaumou R, Solas C, Bourgarel-Rey V, Quaranta S, Rome A, Simon N et al (2011) Impact of plasma and intracellular exposure and CYP3A4, CYP3A5, and ABCB1 genetic polymorphisms on vincristine-induced neurotoxicity. Cancer Chemother Pharmacol 68(6):1633–1638. https://doi.org/10.1007/s00280-011-1745-2
Plasschaert SL, Groninger E, Boezen M, Kema I, de Vries EG, Uges D et al (2004) Influence of functional polymorphisms of the MDR1 gene on vincristine pharmacokinetics in childhood acute lymphoblastic leukemia. Clin Pharmacol Ther 76(3):220–229. https://doi.org/10.1016/j.clpt.2004.05.007
Nakagawa J, Takahata T, Hyodo R, Chen Y, Hasui K, Sasaki K et al (2021) Evaluation for pharmacokinetic exposure of cytotoxic anticancer drugs in elderly patients receiving (R-)CHOP therapy. Sci Rep 11(1):785. https://doi.org/10.1038/s41598-020-80706-2
Moriyama B, Henning SA, Leung J, Falade-Nwulia O, Jarosinski P, Penzak SR, Walsh TJ (2012) Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses 55(4):290–297. https://doi.org/10.1111/j.1439-0507.2011.02158.x
Villikka K, Kivistö KT, Mäenpää H, Joensuu H, Neuvonen PJ (1999) Cytochrome P450-inducing antiepileptics increase the clearance of vincristine in patients with brain tumors. Clin Pharmacol Ther 66(6):589–593. https://doi.org/10.1053/cp.1999.v66.103403001
Xie HG, Wood AJ, Kim RB, Stein CM, Wilkinson GR (2004) Genetic variability in CYP3A5 and its possible consequences. Pharmacogenomics 5(3):243–272. https://doi.org/10.1517/phgs.5.3.243.29833
Hiratsuka M, Takekuma Y, Endo N, Narahara K, Hamdy SI, Kishikawa Y et al (2002) Allele and genotype frequencies of CYP2B6 and CYP3A5 in the Japanese population. Eur J Clin Pharmacol 58(6):417–421. https://doi.org/10.1007/s00228-002-0499-5
Dennison JB, Kulanthaivel P, Barbuch RJ, Renbarger JL, Ehlhardt WJ, Hall SD (2006) Selective metabolism of vincristine in vitro by CYP3A5. Drug Metab Dispos 34(8):1317–1327. https://doi.org/10.1124/dmd.106.009902
Barnett S, Hellmann F, Parke E, Makin G, Tweddle DA, Osborne C et al (2022) Vincristine dosing, drug exposure and therapeutic drug monitoring in neonate and infant cancer patients. Eur J Cancer 164:127–136. https://doi.org/10.1016/j.ejca.2021.09.014
Ceppi F, Langlois-Pelletier C, Gagné V, Rousseau J, Ciolino C, De Lorenzo S et al (2014) Polymorphisms of the vincristine pathway and response to treatment in children with childhood acute lymphoblastic leukemia. Pharmacogenomics 15(8):1105–1116. https://doi.org/10.2217/pgs.14.68
Wolking S, Schaeffeler E, Lerche H, Schwab M, Nies AT (2015) Impact of genetic polymorphisms of ABCB1 (MDR1, P-glycoprotein) on drug disposition and potential clinical implications: update of the literature. Clin Pharmacokinet 54(7):709–735. https://doi.org/10.1007/s40262-015-0267-1
Shu W, Chen L, Hu X, Zhang M, Chen W, Ma L et al (2017) Cytochrome P450 genetic variations can predict mRNA expression, cyclophosphamide 4-hydroxylation, and treatment outcomes in Chinese patients with Non-Hodgkin’s lymphoma. J Clin Pharmacol 57(7):886–898. https://doi.org/10.1002/jcph.878
Egbelakin A, Ferguson MJ, MacGill EA, Lehmann AS, Topletz AR, Quinney SK et al (2011) Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 56(3):361–367. https://doi.org/10.1002/pbc.22845
Funding
The authors did not receive support from any organization for this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, TN; investigation, TT, YC, KS, KK, TT, SY, and KS; measurement of anticancer drugs, J.N. and K.U.; formal analysis, JN; writing—original draft preparation, JN; writing—review and editing, AS, HS, and TN. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Hirosaki University Graduate School of Medicine (project identification code: 2018-055) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in this study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakagawa, J., Takahata, T., Chen, Y. et al. Influence of CYP3A5 and ABCB1 polymorphisms on the pharmacokinetics of vincristine in adult patients receiving CHOP therapy. Cancer Chemother Pharmacol 92, 391–398 (2023). https://doi.org/10.1007/s00280-023-04580-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04580-1