Abstract
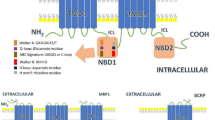
ATP-binding cassette transporter B1 (ABCB1; P-glycoprotein; multidrug resistance protein 1) is an adenosine triphosphate (ATP)-dependent efflux transporter located in the plasma membrane of many different cell types. Numerous structurally unrelated compounds, including drugs and environmental toxins, have been identified as substrates. ABCB1 limits the absorption of xenobiotics from the gut lumen, protects sensitive tissues (e.g. the brain, fetus and testes) from xenobiotics and is involved in biliary and renal secretion of its substrates. In recent years, a large number of polymorphisms of the ABCB1 [ATP-binding cassette, sub-family B (MDR/TAP), member 1] gene have been described. The variants 1236C>T (rs1128503, p.G412G), 2677G>T/A (rs2032582, p.A893S/T) and 3435C>T (rs1045642, p.I1145I) occur at high allele frequencies and create a common haplotype; therefore, they have been most widely studied. This review provides an overview of clinical studies published between 2002 and March 2015. In summary, the effect of ABCB1 variation on P-glycoprotein expression (messenger RNA and protein expression) and/or activity in various tissues (e.g. the liver, gut and heart) appears to be small. Although polymorphisms and haplotypes of ABCB1 have been associated with alterations in drug disposition and drug response, including adverse events with various ABCB1 substrates in different ethnic populations, the results have been majorly conflicting, with limited clinical relevance. Future research activities are warranted, considering a deep-sequencing approach, as well as well-designed clinical studies with appropriate sample sizes to elucidate the impact of rare ABCB1 variants and their potential consequences for effect sizes.


Similar content being viewed by others
References
Bruhn O, Cascorbi I. Polymorphisms of the drug transporters ABCB1, ABCG2, ABCC2 and ABCC3 and their impact on drug bioavailability and clinical relevance. Expert Opin Drug Metab Toxicol. 2014;10:1337–54.
Ieiri I. Functional significance of genetic polymorphisms in P-glycoprotein (MDR1, ABCB1) and breast cancer resistance protein (BCRP, ABCG2). Drug Metab Pharmacokinet. 2012;27:85–105.
Sakaeda T. MDR1 genotype-related pharmacokinetics: fact or fiction? Drug Metab Pharmacokinet. 2005;20:391–414.
Schwab M, Eichelbaum M, Fromm MF. Genetic polymorphisms of the human MDR1 drug transporter. Annu Rev Pharmacol Toxicol. 2003;43:285–307.
Wolf SJ, Bachtiar M, Wang J, Sim TS, Chong SS, Lee CGL. An update on ABCB1 pharmacogenetics: insights from a 3D model into the location and evolutionary conservation of residues corresponding to SNPs associated with drug pharmacokinetics. Pharmacogenomics J. 2011;11:315–25.
Kimchi-Sarfaty C, Oh JM, Kim I-W, Sauna ZE, Calcagno AM, Ambudkar SV, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–8.
Clay AT, Sharom FJ. Multidrug resistance protein: P-glycoprotein. In: You G, Morris ME, editors. Drug transporters molecular characterization and role in drug disposition. Hoboken, New Jersey: Wiley; 2014. p. 141–60.
Cascorbi I. P-glycoprotein: tissue distribution, substrates, and functional consequences of genetic variations. Handb Exp Pharmacol. 2011;201:261–83.
Ieiri I, Takane H, Otsubo K. The MDR1 (ABCB1) gene polymorphism and its clinical implications. Clin Pharmacokinet. 2004;43:553–76.
Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, et al. Internal duplication and homology with bacterial transport proteins in the MDR1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–9.
Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009.
Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–8.
Tatsuta T, Naito M, Oh-hara T, Sugawara I, Tsuruo T. Functional involvement of P-glycoprotein in blood–brain barrier. J Biol Chem. 1992;267:20383–91.
Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood–brain barrier sites. Proc Natl Acad Sci USA. 1989;86:695–8.
Mayer U, Wagenaar E, Beijnen JH, Smit JW, Meijer DK, van Asperen J, et al. Substantial excretion of digoxin via the intestinal mucosa and prevention of long-term digoxin accumulation in the brain by the MDR 1a P-glycoprotein. Br J Pharmacol. 1996;119:1038–44.
Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, et al. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–94.
Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood–brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–24.
Svirnovski AI, Shman TV, Serhiyenka TF, Savitski VP, Smolnikova VV, Fedasenka UU. ABCB1 and ABCG2 proteins, their functional activity and gene expression in concert with drug sensitivity of leukemia cells. Hematology (Amsterdam, Netherlands). 2009;14:204–12.
Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci USA. 1987;84:265–9.
Chandler B, Detsika M, Khoo SH, Williams J, Back DJ, Owen A. Factors impacting the expression of membrane-bound proteins in lymphocytes from HIV-positive subjects. J Antimicrob Chemother. 2007;60:685–9.
Riordan JR, Deuchars K, Kartner N, Alon N, Trent J, Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature. 1985;316:817–9.
Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–98.
Schinkel AH, Kemp S, Dollé M, Rudenko G, Wagenaar E. N-glycosylation and deletion mutants of the human MDR1 P-glycoprotein. J Biol Chem. 1993;268:7474–81.
Gribar JJ, Ramachandra M, Hrycyna CA, Dey S, Ambudkar SV. Functional characterization of glycosylation-deficient human P-glycoprotein using a vaccinia virus expression system. J Membr Biol. 2000;173:203–14.
Germann UA, Chambers TC, Ambudkar SV, Licht T, Cardarelli CO, Pastan I, et al. Characterization of phosphorylation-defective mutants of human P-glycoprotein expressed in mammalian cells. J Biol Chem. 1996;271:1708–16.
Idriss HT, Hannun YA, Boulpaep E, Basavappa S. Regulation of volume-activated chloride channels by P-glycoprotein: phosphorylation has the final say! J Physiol (Lond). 2000;524(Pt 3):629–36.
Lelong-Rebel IH, Cardarelli CO. Differential phosphorylation patterns of P-glycoprotein reconstituted into a proteoliposome system: insight into additional unconventional phosphorylation sites. Anticancer Res. 2005;25:3925–35.
Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–22.
Raviv Y, Pollard HB, Bruggemann EP, Pastan I, Gottesman MM. Photosensitized labeling of a functional multidrug transporter in living drug-resistant tumor cells. J Biol Chem. 1990;265:3975–80.
Higgins CF, Gottesman MM. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992;17:18–21.
Ramachandra M, Ambudkar SV, Chen D, Hrycyna CA, Dey S, Gottesman MM, et al. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry. 1998;37:5010–9.
Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427.
Gatlik-Landwojtowicz E, Aänismaa P, Seelig A. Quantification and characterization of P-glycoprotein-substrate interactions. Biochemistry. 2006;45:3020–32.
Eichelbaum M, Fromm MF, Schwab M. Clinical aspects of the MDR1 (ABCB1) gene polymorphism. Ther Drug Monit. 2004;26:180–5.
Cascorbi I, Haenisch S. Pharmacogenetics of ATP-binding cassette transporters and clinical implications. Methods Mol Biol. 2010;596:95–121.
Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276:14581–7.
Johne A, Brockmöller J, Bauer S, Maurer A, Langheinrich M, Roots I. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66:338–45.
Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993;268:6077–80.
Tanigawara Y, Okamura N, Hirai M, Yasuhara M, Ueda K, Kioka N, et al. Transport of digoxin by human P-glycoprotein expressed in a porcine kidney epithelial cell line (LLC-PK1). J Pharmacol Exp Ther. 1992;263:840–5.
Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–8.
Kurata Y, Ieiri I, Kimura M, Morita T, Irie S, Urae A, et al. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin Pharmacol Ther. 2002;72:209–19.
Verstuyft C, Strabach S, El-Morabet H, Kerb R, Brinkmann U, Dubert L, et al. Dipyridamole enhances digoxin bioavailability via P-glycoprotein inhibition. Clin Pharmacol Ther. 2003;73:51–60.
Johne A, Köpke K, Gerloff T, Mai I, Rietbrock S, Meisel C, et al. Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin Pharmacol Ther. 2002;72:584–94.
Sakaeda T, Nakamura T, Horinouchi M, Kakumoto M, Ohmoto N, Sakai T, et al. MDR1 genotype-related pharmacokinetics of digoxin after single oral administration in healthy Japanese subjects. Pharm Res. 2001;18:1400–4.
Horinouchi M, Sakaeda T, Nakamura T, Morita Y, Tamura T, Aoyama N, et al. Significant genetic linkage of MDR1 polymorphisms at positions 3435 and 2677: functional relevance to pharmacokinetics of digoxin. Pharm Res. 2002;19:1581–5.
Chowbay B, Li H, David M, Cheung YB, Lee EJD. Meta-analysis of the influence of MDR1 C3435T polymorphism on digoxin pharmacokinetics and MDR1 gene expression. Br J Clin Pharmacol. 2005;60:159–71.
Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112:457–73.
1000 Genomes Project Consortium, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65.
Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP), Seattle, WA, USA [Internet]. Available from: http://evs.gs.washington.edu/EVS/. Accessed 21 Jan 2015.
Crouthamel MH, Wu D, Yang Z, Ho RJY. A novel MDR1 G1199T variant alters drug resistance and efflux transport activity of P-glycoprotein in recombinant Hek cells. J Pharm Sci. 2006;95:2767–77.
Jeong H, Herskowitz I, Kroetz DL, Rine J. Function-altering SNPs in the human multidrug transporter gene ABCB1 identified using a Saccharomyces-based assay. PLoS Genet. 2007;3:e39.
Gow JM, Hodges LM, Chinn LW, Kroetz DL. Substrate-dependent effects of human ABCB1 coding polymorphisms. J Pharmacol Exp Ther. 2008;325:435–42.
Ramsey LB, Bruun GH, Yang W, Treviño LR, Vattathil S, Scheet P, et al. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res. 2012;22:1–8.
Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta. 2009;1794:860–71.
Ameyaw MM, Regateiro F, Li T, Liu X, Tariq M, Mobarek A, et al. MDR1 pharmacogenetics: frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001;11:217–21.
Cascorbi I, Gerloff T, Johne A, Meisel C, Hoffmeyer S, Schwab M, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther. 2001;69:169–74.
Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–99.
Lee SS, Kim S-Y, Kim W-Y, Thi-Le H, Yoon Y-R, Yea SS, et al. MDR1 genetic polymorphisms and comparison of MDR1 haplotype profiles in Korean and Vietnamese populations. Ther Drug Monit. 2005;27:531–5.
Schaeffeler E, Eichelbaum M, Brinkmann U, Penger A, Asante-Poku S, Zanger UM, et al. Frequency of C3435T polymorphism of MDR1 gene in African people. Lancet. 2001;358:383–4.
Yamauchi A, Ieiri I, Kataoka Y, Tanabe M, Nishizaki T, Oishi R, et al. Neurotoxicity induced by tacrolimus after liver transplantation: relation to genetic polymorphisms of the ABCB1 (MDR1) gene. Transplantation. 2002;74:571–2.
Sai K, Kaniwa N, Itoda M, Saito Y, Hasegawa R, Komamura K, et al. Haplotype analysis of ABCB1/MDR1 blocks in a Japanese population reveals genotype-dependent renal clearance of irinotecan. Pharmacogenetics. 2003;13:741–57.
Tang K, Wong LP, Lee EJD, Chong SS, Lee CGL. Genomic evidence for recent positive selection at the human MDR1 gene locus. Hum Mol Genet. 2004;13:783–97.
Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, et al. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13:481–94.
Leschziner G, Zabaneh D, Pirmohamed M, Owen A, Rogers J, Coffey AJ, et al. Exon sequencing and high resolution haplotype analysis of ABC transporter genes implicated in drug resistance. Pharmacogenet Genomics. 2006;16:439–50.
Wang D, Johnson AD, Papp AC, Kroetz DL, Sadée W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet Genomics. 2005;15:693–704.
Fung KL, Pan J, Ohnuma S, Lund PE, Pixley JN, Kimchi-Sarfaty C, et al. MDR1 synonymous polymorphisms alter transporter specificity and protein stability in a stable epithelial monolayer. Cancer Res. 2014;74:598–608.
Von Richter O, Burk O, Fromm MF, Thon KP, Eichelbaum M, Kivistö KT. Cytochrome P450 3A4 and P-glycoprotein expression in human small intestinal enterocytes and hepatocytes: a comparative analysis in paired tissue specimens. Clin Pharmacol Ther. 2004;75:172–83.
Schuetz EG, Furuya KN, Schuetz JD. Interindividual variation in expression of P-glycoprotein in normal human liver and secondary hepatic neoplasms. J Pharmacol Exp Ther. 1995;275:1011–8.
Wolbold R, Klein K, Burk O, Nüssler AK, Neuhaus P, Eichelbaum M, et al. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology. 2003;38:978–88.
Meier Y, Pauli-Magnus C, Zanger UM, Klein K, Schaeffeler E, Nüssler AK, et al. Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expression in human liver. Hepatology. 2006;44:62–74.
Prasad B, Evers R, Gupta A, Hop CECA, Salphati L, Shukla S, et al. Interindividual variability in hepatic organic anion-transporting polypeptides and P-glycoprotein (ABCB1) protein expression: quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab Dispos. 2014;42:78–88.
Leschziner GD, Andrew T, Pirmohamed M, Johnson MR. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J. 2007;7:154–79.
Goto M, Masuda S, Saito H, Uemoto S, Kiuchi T, Tanaka K, et al. C3435T polymorphism in the MDR1 gene affects the enterocyte expression level of CYP3A4 rather than Pgp in recipients of living-donor liver transplantation. Pharmacogenetics. 2002;12:451–7.
Siegmund W, Ludwig K, Giessmann T, Dazert P, Schroeder E, Sperker B, et al. The effects of the human MDR1 genotype on the expression of duodenal P-glycoprotein and disposition of the probe drug talinolol. Clin Pharmacol Ther. 2002;72:572–83.
Meissner K, Jedlitschky G, Meyer zu Schwabedissen H, Dazert P, Eckel L, Vogelgesang S, et al. Modulation of multidrug resistance P-glycoprotein 1 (ABCB1) expression in human heart by hereditary polymorphisms. Pharmacogenetics. 2004;14:381–5.
Owen A, Goldring C, Morgan P, Chadwick D, Park BK, Pirmohamed M. Relationship between the C3435T and G2677T(A) polymorphisms in the ABCB1 gene and P-glycoprotein expression in human liver. Br J Clin Pharmacol. 2005;59:365–70.
Uwai Y, Masuda S, Goto M, Motohashi H, Saito H, Okuda M, et al. Common single nucleotide polymorphisms of the MDR1 gene have no influence on its mRNA expression level of normal kidney cortex and renal cell carcinoma in Japanese nephrectomized patients. J Hum Genet. 2004;49:40–5.
Fellay J, Marzolini C, Meaden ER, Back DJ, Buclin T, Chave JP, et al. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359:30–6.
Shou W, Wang D, Zhang K, Wang B, Wang Z, Shi J, et al. Gene-wide characterization of common quantitative trait loci for ABCB1 mRNA expression in normal liver tissues in the chinese population. PLoS One. 2012;7:e46295.
Nakamura T, Sakaeda T, Horinouchi M, Tamura T, Aoyama N, Shirakawa T, et al. Effect of the mutation (C3435T) at exon 26 of the MDR1 gene on expression level of MDR1 messenger ribonucleic acid in duodenal enterocytes of healthy Japanese subjects. Clin Pharmacol Ther. 2002;71:297–303.
Tanabe M, Ieiri I, Nagata N, Inoue K, Ito S, Kanamori Y, et al. Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J Pharmacol Exp Ther. 2001;297:1137–43.
Hitzl M, Schaeffeler E, Hocher B, Slowinski T, Halle H, Eichelbaum M, et al. Variable expression of P-glycoprotein in the human placenta and its association with mutations of the multidrug resistance 1 gene (MDR1, ABCB1). Pharmacogenetics. 2004;14:309–18.
Seedhouse CH, Grundy M, White P, Li Y, Fisher J, Yakunina D, et al. Sequential influences of leukemia-specific and genetic factors on P-glycoprotein expression in blasts from 817 patients entered into the National Cancer Research Network acute myeloid leukemia 14 and 15 trials. Clin Cancer Res. 2007;13:7059–66.
Hodges LM, Markova SM, Chinn LW, Gow JM, Kroetz DL, Klein TE, et al. Very important pharmacogene summary. Pharmacogenet Genomics. 2011;21:152–61.
Elens L, Vandercam B, Yombi J-C, Lison D, Wallemacq P, Haufroid V. Influence of host genetic factors on efavirenz plasma and intracellular pharmacokinetics in HIV-1-infected patients. Pharmacogenomics. 2010;11:1223–34.
Mukonzo JK, Owen JS, Ogwal-Okeng J, Kuteesa RB, Nanzigu S, Sewankambo N, et al. Pharmacogenetic-based efavirenz dose modification: suggestions for an African population and the different CYP2B6 genotypes. PLoS One. 2014;9:e86919.
Ngaimisi E, Habtewold A, Minzi O, Makonnen E, Mugusi S, Amogne W, et al. Importance of ethnicity, CYP2B6 and ABCB1 genotype for efavirenz pharmacokinetics and treatment outcomes: a parallel-group prospective cohort study in two sub-Saharan Africa populations. PLoS One. 2013;8:e67946.
Uhr M, Tontsch A, Namendorf C, Ripke S, Lucae S, Ising M, et al. Polymorphisms in the drug transporter gene ABCB1 predict antidepressant treatment response in depression. Neuron. 2008;57:203–9.
Sarginson JE, Lazzeroni LC, Ryan HS, Ershoff BD, Schatzberg AF, Murphy GM. ABCB1 (MDR1) polymorphisms and antidepressant response in geriatric depression. Pharmacogenet Genomics. 2010;20:467–75.
Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet. 2010;49:141–75.
Hu Y-F, Qiu W, Liu Z-Q, Zhu L-J, Liu Z-Q, Tu J-H, et al. Effects of genetic polymorphisms of CYP3A4, CYP3A5 and MDR1 on cyclosporine pharmacokinetics after renal transplantation. Clin Exp Pharmacol Physiol. 2006;33:1093–8.
Bonhomme-Faivre L, Devocelle A, Saliba F, Chatled S, Maccario J, Farinotti R, et al. MDR-1 C3435T polymorphism influences cyclosporine a dose requirement in liver-transplant recipients. Transplantation. 2004;78:21–5.
Azarpira N, Aghdaie MH, Behzad-Behbahanie A, Geramizadeh B, Behzadi S, Malekhoseinie SA, et al. Association between cyclosporine concentration and genetic polymorphisms of CYP3A5 and MDR1 during the early stage after renal transplantation. Exp Clin Transpl. 2006;4:416–9.
Foote CJ, Greer W, Kiberd BA, Fraser A, Lawen J, Nashan B, et al. MDR1 C3435T polymorphisms correlate with cyclosporine levels in de novo renal recipients. Transpl Proc. 2006;38:2847–9.
Jiang Z-P, Wang Y-R, Xu P, Liu R-R, Zhao X-L, Chen F-P. Meta-analysis of the effect of MDR1 C3435T polymorphism on cyclosporine pharmacokinetics. Basic Clin Pharmacol Toxicol. 2008;103:433–44.
Lee J, Wang R, Yang Y, Lu X, Zhang X, Wang L, et al. The effect of ABCB1 C3435T polymorphism on cyclosporine dose requirements in kidney transplant recipients: a meta-analysis. Basic Clin Pharmacol Toxicol. doi:10.1111/bcpt.12371. Epub 2014 Dec 23.
Crettol S, Venetz J-P, Fontana M, Aubert J-D, Ansermot N, Fathi M, et al. Influence of ABCB1 genetic polymorphisms on cyclosporine intracellular concentration in transplant recipients. Pharmacogenet Genomics. 2008;18:307–15.
Hauser IA, Schaeffeler E, Gauer S, Scheuermann EH, Wegner B, Gossmann J, et al. ABCB1 genotype of the donor but not of the recipient is a major risk factor for cyclosporine-related nephrotoxicity after renal transplantation. J Am Soc Nephrol. 2005;16:1501–11.
Woillard J-B, Rerolle J-P, Picard N, Rousseau A, Guillaudeau A, Munteanu E, et al. Donor P-gp polymorphisms strongly influence renal function and graft loss in a cohort of renal transplant recipients on cyclosporine therapy in a long-term follow-up. Clin Pharmacol Ther. 2010;88:95–100.
Cattaneo D, Ruggenenti P, Baldelli S, Motterlini N, Gotti E, Sandrini S, et al. ABCB1 genotypes predict cyclosporine-related adverse events and kidney allograft outcome. J Am Soc Nephrol. 2009;20:1404–15.
Moore J, McKnight AJ, Dohler B, Simmonds MJ, Courtney AE, Brand OJ, et al. Donor ABCB1 variant associates with increased risk for kidney allograft failure. J Am Soc Nephrol. 2012;23:1891–9.
Fredericks S, Moreton M, Reboux S, Carter ND, Goldberg L, Holt DW, et al. Multidrug resistance gene-1 (MDR-1) haplotypes have a minor influence on tacrolimus dose requirements. Transplantation. 2006;82:705–8.
Loh PT, Lou HX, Zhao Y, Chin YM, Vathsala A. Significant impact of gene polymorphisms on tacrolimus but not cyclosporine dosing in Asian renal transplant recipients. Transpl Proc. 2008;40:1690–5.
Cheung CY, Op den Buijsch RAM, Wong KM, Chan HW, Chau KF, Li CS, et al. Influence of different allelic variants of the CYP3A and ABCB1 genes on the tacrolimus pharmacokinetic profile of Chinese renal transplant recipients. Pharmacogenomics. 2006;7:563–74.
Wang J, Zeevi A, McCurry K, Schuetz E, Zheng H, Iacono A, et al. Impact of ABCB1 (MDR1) haplotypes on tacrolimus dosing in adult lung transplant patients who are CYP3A5 *3/*3 non-expressors. Transpl Immunol. 2006;15:235–40.
Naesens M, Lerut E, de Jonge H, Van Damme B, Vanrenterghem Y, Kuypers DRJ. Donor age and renal P-glycoprotein expression associate with chronic histological damage in renal allografts. J Am Soc Nephrol. 2009;20:2468–80.
Herrlinger KR, Koc H, Winter S, Teml A, Stange EF, Fellermann K, et al. ABCB1 single-nucleotide polymorphisms determine tacrolimus response in patients with ulcerative colitis. Clin Pharmacol Ther. 2011;89:422–8.
Nakajima M, Fujiki Y, Kyo S, Kanaya T, Nakamura M, Maida Y, et al. Pharmacokinetics of paclitaxel in ovarian cancer patients and genetic polymorphisms of CYP2C8, CYP3A4, and MDR1. J Clin Pharmacol. 2005;45:674–82.
Gréen H, Söderkvist P, Rosenberg P, Mirghani RA, Rymark P, Lundqvist EA, et al. Pharmacogenetic studies of paclitaxel in the treatment of ovarian cancer. Basic Clin Pharmacol Toxicol. 2009;104:130–7.
Fransson MN, Gréen H, Litton J-E, Friberg LE. Influence of Cremophor EL and genetic polymorphisms on the pharmacokinetics of paclitaxel and its metabolites using a mechanism-based model. Drug Metab Dispos. 2011;39:247–55.
Jiko M, Yano I, Sato E, Takahashi K, Motohashi H, Masuda S, et al. Pharmacokinetics and pharmacodynamics of paclitaxel with carboplatin or gemcitabine, and effects of CYP3A5 and MDR1 polymorphisms in patients with urogenital cancers. Int J Clin Oncol. 2007;12:284–90.
De Graan A-JM, Elens L, Sprowl JA, Sparreboom A, Friberg LE, van der Holt B, et al. CYP3A4*22 genotype and systemic exposure affect paclitaxel-induced neurotoxicity. Clin Cancer Res. 2013;19:3316–24.
Chang H, Rha SY, Jeung H-C, Im CK, Noh SH, Kim JJ, et al. Association of the ABCB1 3435C>T polymorphism and treatment outcomes in advanced gastric cancer patients treated with paclitaxel-based chemotherapy. Oncol Rep. 2010;23:271–8.
Chang H, Rha SY, Jeung H-C, Im C-K, Ahn JB, Kwon WS, et al. Association of the ABCB1 gene polymorphisms 2677G>T/A and 3435C>T with clinical outcomes of paclitaxel monotherapy in metastatic breast cancer patients. Ann Oncol. 2009;20:272–7.
Sissung TM, Mross K, Steinberg SM, Behringer D, Figg WD, Sparreboom A, et al. Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer. 2006;42:2893–6.
Hertz DL, Motsinger-Reif AA, Drobish A, Winham SJ, McLeod HL, Carey LA, et al. CYP2C8*3 predicts benefit/risk profile in breast cancer patients receiving neoadjuvant paclitaxel. Breast Cancer Res Treat. 2012;134:401–10.
Marsh S, Paul J, King CR, Gifford G, McLeod HL, Brown R. Pharmacogenetic assessment of toxicity and outcome after platinum plus taxane chemotherapy in ovarian cancer: the Scottish Randomised Trial in Ovarian Cancer. J Clin Oncol. 2007;25:4528–35.
Fajac A, Gligorov J, Rezai K, Lévy P, Lévy E, Selle F, et al. Effect of ABCB1 C3435T polymorphism on docetaxel pharmacokinetics according to menopausal status in breast cancer patients. Br J Cancer. 2010;103:560–6.
Sissung TM, Baum CE, Deeken J, Price DK, Aragon-Ching J, Steinberg SM, et al. ABCB1 genetic variation influences the toxicity and clinical outcome of patients with androgen-independent prostate cancer treated with docetaxel. Clin Cancer Res. 2008;14:4543–9.
Tsai S-M, Lin C-Y, Wu S-H, Hou LA, Ma H, Tsai L-Y, et al. Side effects after docetaxel treatment in Taiwanese breast cancer patients with CYP3A4, CYP3A5, and ABCB1 gene polymorphisms. Clin Chim Acta. 2009;404:160–5.
Choi JR, Kim J-O, Kang DR, Shin J-Y, Zhang XH, Oh JE, et al. Genetic variations of drug transporters can influence on drug response in patients treated with docetaxel chemotherapy. Cancer Res Treat. doi:10.4143/crt.2014.012. Epub 2014 Dec 16.
Han J-Y, Lim H-S, Yoo Y-K, Shin ES, Park YH, Lee SY, et al. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2007;110:138–47.
De Mattia E, Toffoli G, Polesel J, D’Andrea M, Corona G, Zagonel V, et al. Pharmacogenetics of ABC and SLC transporters in metastatic colorectal cancer patients receiving first-line FOLFIRI treatment. Pharmacogenet Genomics. 2013;23:549–57.
Lara PN, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530–5.
Mahon F-X, Belloc F, Lagarde V, Chollet C, Moreau-Gaudry F, Reiffers J, et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood. 2003;101:2368–73.
Gurney H, Wong M, Balleine RL, Rivory LP, McLachlan AJ, Hoskins JM, et al. Imatinib disposition and ABCB1 (MDR1, P-glycoprotein) genotype. Clin Pharmacol Ther. 2007;82:33–40.
Deenik W, van der Holt B, Janssen JJWM, Chu IWT, Valk PJM, Ossenkoppele GJ, et al. Polymorphisms in the multidrug resistance gene MDR1 (ABCB1) predict for molecular resistance in patients with newly diagnosed chronic myeloid leukemia receiving high-dose imatinib. Blood. 2010;116:6144–5 Author reply 6145–6.
Ni L-N, Li J-Y, Miao K-R, Qiao C, Zhang S-J, Qiu H-R, et al. Multidrug resistance gene (MDR1) polymorphisms correlate with imatinib response in chronic myeloid leukemia. Med Oncol. 2011;28:265–9.
Dulucq S, Bouchet S, Turcq B, Lippert E, Etienne G, Reiffers J, et al. Multidrug resistance gene (MDR1) polymorphisms are associated with major molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2008;112:2024–7.
Elghannam DM, Ibrahim L, Ebrahim MA, Azmy E, Hakem H. Association of MDR1 gene polymorphism (G2677T) with imatinib response in Egyptian chronic myeloid leukemia patients. Hematology. 2014;19:123–8.
Van der Holt B, Van den Heuvel-Eibrink MM, Van Schaik RHN, van der Heiden IP, Wiemer EAC, Vossebeld PJM, et al. ABCB1 gene polymorphisms are not associated with treatment outcome in elderly acute myeloid leukemia patients. Clin Pharmacol Ther. 2006;80:427–39.
Kim DH, Park JY, Sohn SK, Lee NY, Baek JH, Jeon SB, et al. Multidrug resistance-1 gene polymorphisms associated with treatment outcomes in de novo acute myeloid leukemia. Int J Cancer. 2006;118:2195–201.
Hur E-H, Lee J-H, Lee MJ, Choi S-J, Lee J-H, Kang MJ, et al. C3435T polymorphism of the MDR1 gene is not associated with P-glycoprotein function of leukemic blasts and clinical outcome in patients with acute myeloid leukemia. Leuk Res. 2008;32:1601–4.
Buda G, Maggini V, Galimberti S, Martino A, Giuliani N, Morabito F, et al. MDR1 polymorphism influences the outcome of multiple myeloma patients. Br J Haematol. 2007;137:454–6.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90.
Mizuno T, Fukudo M, Terada T, Kamba T, Nakamura E, Ogawa O, et al. Impact of genetic variation in breast cancer resistance protein (BCRP/ABCG2) on sunitinib pharmacokinetics. Drug Metab Pharmacokinet. 2012;27:631–9.
Beuselinck B, Karadimou A, Lambrechts D, Claes B, Wolter P, Couchy G, et al. Single-nucleotide polymorphisms associated with outcome in metastatic renal cell carcinoma treated with sunitinib. Br J Cancer. 2013;108:887–900.
Beuselinck B, Lambrechts D, Van Brussel T, Wolter P, Cardinaels N, Joniau S, et al. Efflux pump ABCB1 single nucleotide polymorphisms and dose reductions in patients with metastatic renal cell carcinoma treated with sunitinib. Acta Oncol. 2014;53:1413–22.
Garcia-Donas J, Esteban E, Leandro-García LJ, Castellano DE, del Alba AG, Climent MA, et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: a multicentre, observational, prospective study. Lancet Oncol. 2011;12:1143–50.
Van Erp NP, Eechoute K, van der Veldt AA, Haanen JB, Reyners AKL, Mathijssen RHJ, et al. Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J Clin Oncol. 2009;27:4406–12.
Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–5.
Mukonzo JK, Röshammar D, Waako P, Andersson M, Fukasawa T, Milani L, et al. A novel polymorphism in ABCB1 gene, CYP2B6*6 and sex predict single-dose efavirenz population pharmacokinetics in Ugandans. Br J Clin Pharmacol. 2009;68:690–9.
Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–41.
Stöhr W, Back D, Dunn D, Sabin C, Winston A, Gilson R, et al. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther (Lond). 2008;13:675–85.
Burger D, van der Heiden I, la Porte C, van der Ende M, Groeneveld P, Richter C, et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol. 2006;61:148–54.
Park WB, Choe PG, Song K-H, Jeon JH, Park SW, Kim HB, et al. Genetic factors influencing severe atazanavir-associated hyperbilirubinemia in a population with low UDP-glucuronosyltransferase 1A1*28 allele frequency. Clin Infect Dis. 2010;51:101–6.
Haas DW, Bartlett JA, Andersen JW, Sanne I, Wilkinson GR, Hinkle J, et al. Pharmacogenetics of nevirapine-associated hepatotoxicity: an Adult AIDS Clinical Trials Group collaboration. Clin Infect Dis. 2006;43:783–6.
Ciccacci C, Borgiani P, Ceffa S, Sirianni E, Marazzi MC, Altan AMD, et al. Nevirapine-induced hepatotoxicity and pharmacogenetics: a retrospective study in a population from Mozambique. Pharmacogenomics. 2010;11:23–31.
Colombo S, Soranzo N, Rotger M, Sprenger R, Bleiber G, Furrer H, et al. Influence of ABCB1, ABCC1, ABCC2, and ABCG2 haplotypes on the cellular exposure of nelfinavir in vivo. Pharmacogenet Genomics. 2005;15:599–608.
Saitoh A, Capparelli E, Aweeka F, Sarles E, Singh KK, Kovacs A, et al. CYP2C19 genetic variants affect nelfinavir pharmacokinetics and virologic response in HIV-1-infected children receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;54:285–9.
Winzer R, Langmann P, Zilly M, Tollmann F, Schubert J, Klinker H, et al. No influence of the P-glycoprotein genotype (MDR1 C3435T) on plasma levels of lopinavir and efavirenz during antiretroviral treatment. Eur J Med Res. 2003;8:531–4.
Ma Q, Brazeau D, Zingman BS, Reichman RC, Fischl MA, Gripshover BM, et al. Multidrug resistance 1 polymorphisms and trough concentrations of atazanavir and lopinavir in patients with HIV. Pharmacogenomics. 2007;8:227–35.
Fröhlich M, Burhenne J, Martin-Facklam M, Weiss J, von Wolff M, Strowitzki T, et al. Oral contraception does not alter single dose saquinavir pharmacokinetics in women. Br J Clin Pharmacol. 2004;57:244–52.
Solas C, Simon N, Drogoul M-P, Quaranta S, Frixon-Marin V, Bourgarel-Rey V, et al. Minimal effect of MDR1 and CYP3A5 genetic polymorphisms on the pharmacokinetics of indinavir in HIV-infected patients. Br J Clin Pharmacol. 2007;64:353–62.
Curras V, Hocht C, Mangano A, Niselman V, Mariño Hernández E, Cáceres Guido P, et al. Pharmacokinetic study of the variability of indinavir drug levels when boosted with ritonavir in HIV-infected children. Pharmacology. 2009;83:59–66.
Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–33.
Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75.
Zuern CS, Schwab M, Gawaz M, Geisler T. Platelet pharmacogenomics. J Thromb Haemost. 2010;8:1147–58.
Geisler T, Bigalke B, Schwab M. CYP2C19 genotype and outcomes of clopidogrel treatment. N Engl J Med. 2011;364:481 Author reply 482.
Taubert D, von Beckerath N, Grimberg G, Lazar A, Jung N, Goeser T, et al. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006;80:486–501.
Von Beckerath N, Taubert D, Pogatsa-Murray G, Schömig E, Kastrati A, Schömig A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) trial. Circulation. 2005;112:2946–50.
Karaźniewicz-Łada M, Danielak D, Rubiś B, Burchardt P, Komosa A, Lesiak M, et al. Impact of common ABCB1 polymorphism on pharmacokinetics and pharmacodynamics of clopidogrel and its metabolites. J Clin Pharm Ther. 2015;40:226–31.
Frelinger AL, Bhatt DL, Lee RD, Mulford DJ, Wu J, Nudurupati S, et al. Clopidogrel pharmacokinetics and pharmacodynamics vary widely despite exclusion or control of polymorphisms (CYP2C19, ABCB1, PON1), noncompliance, diet, smoking, co-medications (including proton pump inhibitors), and pre-existent variability in platelet function. J Am Coll Cardiol. 2013;61:872–9.
Verschuren JJW, Boden H, Wessels JAM, van der Hoeven BL, Trompet S, Heijmans BT, et al. Value of platelet pharmacogenetics in common clinical practice of patients with ST-segment elevation myocardial infarction. Int J Cardiol. 2013;167:2882–8.
Xie C, Ding X, Gao J, Wang H, Hang Y, Zhang H, et al. The effects of CES1A2 A(−816)C and CYP2C19 loss-of-function polymorphisms on clopidogrel response variability among Chinese patients with coronary heart disease. Pharmacogenet Genomics. 2014;24:204–10.
Carlquist JF, Knight S, Horne BD, Huntinghouse JA, Rollo JS, Muhlestein JB, et al. Cardiovascular risk among patients on clopidogrel anti-platelet therapy after placement of drug-eluting stents is modified by genetic variants in both the CYP2C19 and ABCB1 genes. Thromb Haemost. 2013;109:744–54.
Su J, Xu J, Li X, Zhang H, Hu J, Fang R, et al. ABCB1 C3435T polymorphism and response to clopidogrel treatment in coronary artery disease (CAD) patients: a meta-analysis. PLoS One. 2012;7:e46366.
Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376:1312–9.
Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–8.
Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9.
Löscher W. How to explain multidrug resistance in epilepsy? Epilepsy Curr. 2005;5:107–12.
Aronica E, Sisodiya SM, Gorter JA. Cerebral expression of drug transporters in epilepsy. Adv Drug Deliv Rev. 2012;64:919–29.
Tishler DM, Weinberg KI, Hinton DR, Barbaro N, Annett GM, Raffel C. MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia. 1995;36:1–6.
Löscher W, Potschka H. Blood–brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98.
Löscher W, Potschka H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharmacol Exp Ther. 2002;301:7–14.
Siddiqui A, Kerb R, Weale ME, Brinkmann U, Smith A, Goldstein DB, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348:1442–8.
Tan NCK, Heron SE, Scheffer IE, Pelekanos JT, McMahon JM, Vears DF, et al. Failure to confirm association of a polymorphism in ABCB1 with multidrug-resistant epilepsy. Neurology. 2004;63:1090–2.
Sánchez MB, Herranz JL, Leno C, Arteaga R, Oterino A, Valdizán EM, et al. Genetic factors associated with drug-resistance of epilepsy: relevance of stratification by patient age and aetiology of epilepsy. Seizure. 2010;19:93–101.
Leschziner G, Jorgensen AL, Andrew T, Pirmohamed M, Williamson PR, Marson AG, et al. Clinical factors and ABCB1 polymorphisms in prediction of antiepileptic drug response: a prospective cohort study. Lancet Neurol. 2006;5:668–76.
Dong L, Luo R, Tong Y, Cai X, Mao M, Yu D. Lack of association between ABCB1 gene polymorphisms and pharmacoresistant epilepsy: an analysis in a western Chinese pediatric population. Brain Res. 2011;1391:114–24.
Alpman A, Ozkinay F, Tekgul H, Gokben S, Pehlivan S, Schalling M, et al. Multidrug resistance 1 (MDR1) gene polymorphisms in childhood drug-resistant epilepsy. J Child Neurol. 2010;25:1485–90.
Emich-Widera E, Likus W, Kazek B, Niemiec P, Balcerzyk A, Sieroń AL, et al. CYP3A5*3 and C3435T MDR1 polymorphisms in prognostication of drug-resistant epilepsy in children and adolescents. Biomed Res Int. 2013;2013:526837.
Hughes JR. One of the hottest topics in epileptology: ABC proteins. Their inhibition may be the future for patients with intractable seizures. Neurol Res. 2008;30:920–5.
Manna I, Gambardella A, Labate A, Mumoli L, Ferlazzo E, Pucci F, et al. Polymorphism of the multidrug resistance 1 gene MDR1/ABCB1 C3435T and response to antiepileptic drug treatment in temporal lobe epilepsy. Seizure. 2015;24:124–6.
Seo T, Ishitsu T, Ueda N, Nakada N, Yurube K, Ueda K, et al. ABCB1 polymorphisms influence the response to antiepileptic drugs in Japanese epilepsy patients. Pharmacogenomics. 2006;7:551–61.
Bournissen FG, Moretti ME, Juurlink DN, Koren G, Walker M, Finkelstein Y. Polymorphism of the MDR1/ABCB1 C3435T drug-transporter and resistance to anticonvulsant drugs: a meta-analysis. Epilepsia. 2009;50:898–903.
Haerian BS, Roslan H, Raymond AA, Tan CT, Lim KS, Zulkifli SZ, et al. ABCB1 C3435T polymorphism and the risk of resistance to antiepileptic drugs in epilepsy: a systematic review and meta-analysis. Seizure. 2010;19:339–46.
Haerian BS, Lim KS, Tan CT, Raymond AA, Mohamed Z. Association of ABCB1 gene polymorphisms and their haplotypes with response to antiepileptic drugs: a systematic review and meta-analysis. Pharmacogenomics. 2011;12:713–25.
Puranik YG, Birnbaum AK, Marino SE, Ahmed G, Cloyd JC, Remmel RP, et al. Association of carbamazepine major metabolism and transport pathway gene polymorphisms and pharmacokinetics in patients with epilepsy. Pharmacogenomics. 2013;14:35–45.
Meng H, Guo G, Ren J, Zhou H, Ge Y, Guo Y. Effects of ABCB1 polymorphisms on plasma carbamazepine concentrations and pharmacoresistance in Chinese patients with epilepsy. Epilepsy Behav. 2011;21:27–30.
Zhu X, Yun W, Sun X, Qiu F, Zhao L, Guo Y. Effects of major transporter and metabolizing enzyme gene polymorphisms on carbamazepine metabolism in Chinese patients with epilepsy. Pharmacogenomics. 2014;15:1867–79.
Subenthiran S, Abdullah NR, Muniandy PK, Joseph JP, Cheong KC, Ismail Z, et al. G2677T polymorphism can predict treatment outcome of Malaysians with complex partial seizures being treated with carbamazepine. Genet Mol Res. 2013;12:5937–44.
Ozgon GO, Bebek N, Gul G, Cine N. Association of MDR1 (C3435T) polymorphism and resistance to carbamazepine in epileptic patients from Turkey. Eur Neurol. 2008;59:67–70.
Ponnala S, Chaudhari JR, Jaleel MA, Bhiladvala D, Kaipa PR, Das UN, et al. Role of MDR1 C3435T and GABRG2 C588T gene polymorphisms in seizure occurrence and MDR1 effect on anti-epileptic drug (phenytoin) absorption. Genet Test Mol Biomarkers. 2012;16:550–7.
Ebid A-HIM, Ahmed MMM, Mohammed SA. Therapeutic drug monitoring and clinical outcomes in epileptic Egyptian patients: a gene polymorphism perspective study. Ther Drug Monit. 2007;29:305–12.
Turgut G, Kurt E, Sengul C, Alatas G, Kursunluoglu R, Oral T, et al. Association of MDR1 C3435T polymorphism with bipolar disorder in patients treated with valproic acid. Mol Biol Rep. 2009;36:495–9.
Haerian BS, Lim KS, Tan HJ, Mohamed EHM, Tan CT, Raymond AA, et al. Association between ABCB1 polymorphism and response to sodium valproate treatment in Malaysian epilepsy patients. Epileptic Disord. 2011;13:65–75.
Lovrić M, Božina N, Hajnšek S, Kuzman MR, Sporiš D, Lalić Z, et al. Association between lamotrigine concentrations and ABCB1 polymorphisms in patients with epilepsy. Ther Drug Monit. 2012;34:518–25.
Baltes S, Fedrowitz M, Tortós CL, Potschka H, Löscher W. Valproic acid is not a substrate for P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of in vitro and in vivo transport assays. J Pharmacol Exp Ther. 2007;320:331–43.
Baltes S, Gastens AM, Fedrowitz M, Potschka H, Kaever V, Löscher W. Differences in the transport of the antiepileptic drugs phenytoin, levetiracetam and carbamazepine by human and mouse P-glycoprotein. Neuropharmacology. 2007;52:333–46.
Mosyagin I, Runge U, Schroeder HW, Dazert E, Vogelgesang S, Siegmund W, et al. Association of ABCB1 genetic variants 3435C>T and 2677G>T to ABCB1 mRNA and protein expression in brain tissue from refractory epilepsy patients. Epilepsia. 2008;49:1555–61.
Cascorbi I. ABC transporters in drug-refractory epilepsy: limited clinical significance of pharmacogenetics? Clin Pharmacol Ther. 2010;87:15–8.
Löscher W, Sills GJ. Drug resistance in epilepsy: why is a simple explanation not enough? Epilepsia. 2007;48:2370–2.
Mirza N, Vasieva O, Marson AG, Pirmohamed M. Exploring the genomic basis of pharmacoresistance in epilepsy: an integrative analysis of large-scale gene expression profiling studies on brain tissue from epilepsy surgery. Hum Mol Genet. 2011;20:4381–94.
Weber YG, Nies AT, Schwab M, Lerche H. Genetic biomarkers in epilepsy. Neurotherapeutics. 2014;11:324–33.
Trivedi MH, Rush AJ, Wisniewski SR, Warden D, McKinney W, Downing M, et al. Factors associated with health-related quality of life among outpatients with major depressive disorder: a STAR*D report. J Clin Psychiatry. 2006;67:185–95.
Thuerauf N, Fromm MF. The role of the transporter P-glycoprotein for disposition and effects of centrally acting drugs and for the pathogenesis of CNS diseases. Eur Arch Psychiatry Clin Neurosci. 2006;256:281–6.
Uhr M, Grauer MT, Holsboer F. Differential enhancement of antidepressant penetration into the brain in mice with abcb1ab (mdr1ab) P-glycoprotein gene disruption. Biol Psychiatry. 2003;54:840–6.
Uhr M, Grauer MT. abcb1ab P-glycoprotein is involved in the uptake of citalopram and trimipramine into the brain of mice. J Psychiatr Res. 2003;37:179–85.
Linnet K, Ejsing TB. A review on the impact of P-glycoprotein on the penetration of drugs into the brain. Focus on psychotropic drugs. Eur Neuropsychopharmacol. 2008;18:157–69.
Lin K-M, Chiu Y-F, Tsai I-J, Chen C-H, Shen WW, Liu SC, et al. ABCB1 gene polymorphisms are associated with the severity of major depressive disorder and its response to escitalopram treatment. Pharmacogenet Genomics. 2011;21:163–70.
Ozbey G, Yucel B, Taycan SE, Kan D, Bodur NE, Arslan T, et al. ABCB1 C3435T polymorphism is associated with susceptibility to major depression, but not with a clinical response to citalopram in a Turkish population. Pharmacol Rep. 2014;66:235–8.
Mihaljevic Peles A, Bozina N, Sagud M, Rojnic Kuzman M, Lovric M. MDR1 gene polymorphism: therapeutic response to paroxetine among patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1439–44.
Kato M, Fukuda T, Serretti A, Wakeno M, Okugawa G, Ikenaga Y, et al. ABCB1 (MDR1) gene polymorphisms are associated with the clinical response to paroxetine in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:398–404.
Yasui-Furukori N, Mihara K, Takahata T, Suzuki A, Nakagami T, De Vries R, et al. Effects of various factors on steady-state plasma concentrations of risperidone and 9-hydroxyrisperidone: lack of impact of MDR-1 genotypes. Br J Clin Pharmacol. 2004;57:569–75.
Kastelic M, Koprivsek J, Plesnicar BK, Serretti A, Mandelli L, Locatelli I, et al. MDR1 gene polymorphisms and response to acute risperidone treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:387–92.
Jovanović N, Božina N, Lovrić M, Medved V, Jakovljević M, Peleš AM. The role of CYP2D6 and ABCB1 pharmacogenetics in drug-naïve patients with first-episode schizophrenia treated with risperidone. Eur J Clin Pharmacol. 2010;66:1109–17.
Gunes A, Spina E, Dahl M-L, Scordo MG. ABCB1 polymorphisms influence steady-state plasma levels of 9-hydroxyrisperidone and risperidone active moiety. Ther Drug Monit. 2008;30:628–33.
Xiang Q, Zhao X, Zhou Y, Duan JL, Cui YM. Effect of CYP2D6, CYP3A5, and MDR1 genetic polymorphisms on the pharmacokinetics of risperidone and its active moiety. J Clin Pharmacol. 2010;50:659–66.
Xing Q, Gao R, Li H, Feng G, Xu M, Duan S, et al. Polymorphisms of the ABCB1 gene are associated with the therapeutic response to risperidone in Chinese schizophrenia patients. Pharmacogenomics. 2006;7:987–93.
Meyer UA, Zanger UM, Schwab M. Omics and drug response. Annu Rev Pharmacol Toxicol. 2013;53:475–502.
Zakim D, Schwab M. Data collection as a barrier to personalized medicine. Trends Pharmacol Sci. 2015;36:68–71.
Fromm MF. Importance of P-glycoprotein at blood–tissue barriers. Trends Pharmacol Sci. 2004;25:423–9.
Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92:414–7.
Anglicheau D, Verstuyft C, Laurent-Puig P, Becquemont L, Schlageter M-H, Cassinat B, et al. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol. 2003;14:1889–96.
Bonhomme-Faivre L, Picard V, Saliba F, Abbara C, Fodil M, Chaunoy M, et al. Effect of the ABCB1 3435C>T polymorphism on tacrolimus concentrations and dosage requirements in liver transplant recipients. Am J Health Syst Pharm. 2009;66:1645–51.
Elens L, Capron A, Kerckhove VV, Lerut J, Mourad M, Lison D, et al. 1199G>A and 2677G>T/A polymorphisms of ABCB1 independently affect tacrolimus concentration in hepatic tissue after liver transplantation. Pharmacogenet Genomics. 2007;17:873–83.
Hawwa AF, McKiernan PJ, Shields M, Millership JS, Collier PS, McElnay JC. Influence of ABCB1 polymorphisms and haplotypes on tacrolimus nephrotoxicity and dosage requirements in children with liver transplant. Br J Clin Pharmacol. 2009;68:413–21.
López-Montenegro Soria MA. Kanter Berga J, Beltrán Catalán S, Milara Payá J, Pallardó Mateu LM, Jiménez Torres NV. Genetic polymorphisms and individualized tacrolimus dosing. Transpl Proc. 2010;42:3031–3.
Macphee IAM, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, et al. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation. 2002;74:1486–9.
Provenzani A, Notarbartolo M, Labbozzetta M, Poma P, Vizzini G, Salis P, et al. Influence of CYP3A5 and ABCB1 gene polymorphisms and other factors on tacrolimus dosing in Caucasian liver and kidney transplant patients. Int J Mol Med. 2011;28:1093–102.
Roy JN, Barama A, Poirier C, Vinet B, Roger M. CYP3A4, CYP3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharmacogenet Genomics. 2006;16:659–65.
Wei-lin W, Jing J, Shu-sen Z, Li-hua Wu, Ting-bo L, Song-feng Y, et al. Tacrolimus dose requirement in relation to donor and recipient ABCB1 and CYP3A5 gene polymorphisms in Chinese liver transplant patients. Liver Transpl. 2006;12:775–80.
Cho J-H, Yoon Y-D, Park J-Y, Song E-J, Choi J-Y, Yoon S-H, et al. Impact of cytochrome P450 3A and ATP-binding cassette subfamily B member 1 polymorphisms on tacrolimus dose-adjusted trough concentrations among Korean renal transplant recipients. Transpl Proc. 2012;44:109–14.
Choi JH, Lee YJ, Jang SB, Lee J-E, Kim KH, Park K. Influence of the CYP3A5 and MDR1 genetic polymorphisms on the pharmacokinetics of tacrolimus in healthy Korean subjects. Br J Clin Pharmacol. 2007;64:185–91.
Díaz-Molina B, Tavira B, Lambert JL, Bernardo MJ, Alvarez V, Coto E. Effect of CYP3A5, CYP3A4, and ABCB1 genotypes as determinants of tacrolimus dose and clinical outcomes after heart transplantation. Transpl Proc. 2012;44:2635–8.
Gervasini G, Garcia M, Macias RM, Cubero JJ, Caravaca F, Benitez J. Impact of genetic polymorphisms on tacrolimus pharmacokinetics and the clinical outcome of renal transplantation. Transpl Int. 2012;25:471–80.
Gómez-Bravo MA, Salcedo M, Fondevila C, Suarez F, Castellote J, Rufian S, et al. Impact of donor and recipient CYP3A5 and ABCB1 genetic polymorphisms on tacrolimus dosage requirements and rejection in Caucasian Spanish liver transplant patients. J Clin Pharmacol. 2013;53:1146–54.
Goto M, Masuda S, Kiuchi T, Ogura Y, Oike F, Okuda M, et al. CYP3A5*1-carrying graft liver reduces the concentration/oral dose ratio of tacrolimus in recipients of living-donor liver transplantation. Pharmacogenetics. 2004;14:471–8.
Haufroid V, Wallemacq P, VanKerckhove V, Elens L, De Meyer M, Eddour DC, et al. CYP3A5 and ABCB1 polymorphisms and tacrolimus pharmacokinetics in renal transplant candidates: guidelines from an experimental study. Am J Transpl. 2006;6:2706–13.
Hesselink DA, van Schaik RHN, van Agteren M, de Fijter JW, Hartmann A, Zeier M, et al. CYP3A5 genotype is not associated with a higher risk of acute rejection in tacrolimus-treated renal transplant recipients. Pharmacogenet Genomics. 2008;18:339–48.
Hosohata K, Masuda S, Yonezawa A, Katsura T, Oike F, Ogura Y, et al. MDR1 haplotypes conferring an increased expression of intestinal CYP3A4 rather than MDR1 in female living-donor liver transplant patients. Pharm Res. 2009;26:1590–5.
Jun KR, Lee W, Jang MS, Chun S, Song G-W, Park KT, et al. Tacrolimus concentrations in relation to CYP3A and ABCB1 polymorphisms among solid organ transplant recipients in Korea. Transplantation. 2009;87:1225–31.
Kuypers DRJ, de Jonge H, Naesens M, Lerut E, Verbeke K, Vanrenterghem Y. CYP3A5 and CYP3A4 but not MDR1 single-nucleotide polymorphisms determine long-term tacrolimus disposition and drug-related nephrotoxicity in renal recipients. Clin Pharmacol Ther. 2007;82:711–25.
Kuypers DRJ, Naesens M, de Jonge H, Lerut E, Verbeke K, Vanrenterghem Y. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther Drug Monit. 2010;32:394–404.
Li D, Zhu J-Y, Gao J, Wang X, Lou Y-Q, Zhang G-L. Polymorphisms of tumor necrosis factor-alpha, interleukin-10, cytochrome P450 3A5 and ABCB1 in Chinese liver transplant patients treated with immunosuppressant tacrolimus. Clin Chim Acta. 2007;383:133–9.
Li D, Lu W, Zhu J-Y, Gao J, Lou Y-Q, Zhang G-L. Population pharmacokinetics of tacrolimus and CYP3A5, MDR1 and IL-10 polymorphisms in adult liver transplant patients. J Clin Pharm Ther. 2007;32:505–15.
MacPhee IAM, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, et al. The influence of pharmacogenetics on the time to achieve target tacrolimus concentrations after kidney transplantation. Am J Transpl. 2004;4:914–9.
Mai I, Perloff ES, Bauer S, Goldammer M, Johne A, Filler G, et al. MDR1 haplotypes derived from exons 21 and 26 do not affect the steady-state pharmacokinetics of tacrolimus in renal transplant patients. Br J Clin Pharmacol. 2004;58:548–53.
Min S-I, Kim SY, Ahn SH, Min S-K, Kim SH, Kim YS, et al. CYP3A5 *1 allele: impacts on early acute rejection and graft function in tacrolimus-based renal transplant recipients. Transplantation. 2010;90:1394–400.
Mourad M, Wallemacq P, De Meyer M, Brandt D, Van Kerkhove V, Malaise J, et al. The influence of genetic polymorphisms of cytochrome P450 3A5 and ABCB1 on starting dose- and weight-standardized tacrolimus trough concentrations after kidney transplantation in relation to renal function. Clin Chem Lab Med. 2006;44:1192–8.
Op den Buijsch RAM, Christiaans MHL, Stolk LML, de Vries JE, Cheung CY, Undre NA, et al. Tacrolimus pharmacokinetics and pharmacogenetics: influence of adenosine triphosphate-binding cassette B1 (ABCB1) and cytochrome (CYP) 3A polymorphisms. Fundam Clin Pharmacol. 2007;21:427–35.
Provenzani A, Notarbartolo M, Labbozzetta M, Poma P, Biondi F, Sanguedolce R, et al. The effect of CYP3A5 and ABCB1 single nucleotide polymorphisms on tacrolimus dose requirements in Caucasian liver transplant patients. Ann Transpl. 2009;14:23–31.
Shi Y, Li Y, Tang J, Zhang J, Zou Y, Cai B, et al. Influence of CYP3A4, CYP3A5 and MDR-1 polymorphisms on tacrolimus pharmacokinetics and early renal dysfunction in liver transplant recipients. Gene. 2013;512:226–31.
Shilbayeh S. The impact of genetic polymorphisms on time required to attain the target tacrolimus levels and subsequent pharmacodynamic outcomes in pediatric kidney transplant patients. Saudi J Kidney Dis Transpl. 2014;25:266–77.
Tada H, Tsuchiya N, Satoh S, Kagaya H, Li Z, Sato K, et al. Impact of CYP3A5 and MDR1(ABCB1) C3435T polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transpl Proc. 2005;37:1730–2.
Tsuchiya N, Satoh S, Tada H, Li Z, Ohyama C, Sato K, et al. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation. 2004;78:1182–7.
Zhang X, Liu Z, Zheng J, Chen Z, Tang Z, Chen J, et al. Influence of CYP3A5 and MDR1 polymorphisms on tacrolimus concentration in the early stage after renal transplantation. Clin Transpl. 2005;19:638–43.
Zheng H, Zeevi A, Schuetz E, Lamba J, McCurry K, Griffith BP, et al. Tacrolimus dosing in adult lung transplant patients is related to cytochrome P4503A5 gene polymorphism. J Clin Pharmacol. 2004;44:135–40.
Mourad M, Mourad G, Wallemacq P, Garrigue V, Van Bellingen C, Van Kerckhove V, et al. Sirolimus and tacrolimus trough concentrations and dose requirements after kidney transplantation in relation to CYP3A5 and MDR1 polymorphisms and steroids. Transplantation. 2005;80:977–84.
Renders L, Frisman M, Ufer M, Mosyagin I, Haenisch S, Ott U, et al. CYP3A5 genotype markedly influences the pharmacokinetics of tacrolimus and sirolimus in kidney transplant recipients. Clin Pharmacol Ther. 2007;81:228–34.
Lemaitre F, Bezian E, Goldwirt L, Fernandez C, Farinotti R, Varnous S, et al. Population pharmacokinetics of everolimus in cardiac recipients: comedications, ABCB1, and CYP3A5 polymorphisms. Ther Drug Monit. 2012;34:686–94.
Bandur S, Petrasek J, Hribova P, Novotna E, Brabcova I, Viklicky O. Haplotypic structure of ABCB1/MDR1 gene modifies the risk of the acute allograft rejection in renal transplant recipients. Transplantation. 2008;86:1206–13.
Singh R, Srivastava A, Kapoor R, Mittal RD. Do drug transporter (ABCB1) SNPs influence cyclosporine and tacrolimus dose requirements and renal allograft outcome in the posttransplantation period? J Clin Pharmacol. 2011;51:603–15.
Haufroid V, Mourad M, Van Kerckhove V, Wawrzyniak J, De Meyer M, Eddour DC, et al. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147–54.
Hesselink DA, van Schaik RHN, van der Heiden IP, van der Werf M, Gregoor PJHS, Lindemans J, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245–54.
Onizuka M, Kunii N, Toyosaki M, Machida S, Ohgiya D, Ogawa Y, et al. Cytochrome P450 genetic polymorphisms influence the serum concentration of calcineurin inhibitors in allogeneic hematopoietic SCT recipients. Bone Marrow Transpl. 2011;46:1113–7.
Anglicheau D, Thervet E, Etienne I, Hurault De Ligny B, Le Meur Y, Touchard G, et al. CYP3A5 and MDR1 genetic polymorphisms and cyclosporine pharmacokinetics after renal transplantation. Clin Pharmacol Ther. 2004;75:422–33.
Chowbay B, Cumaraswamy S, Cheung YB, Zhou Q, Lee EJD. Genetic polymorphisms in MDR1 and CYP3A4 genes in Asians and the influence of MDR1 haplotypes on cyclosporin disposition in heart transplant recipients. Pharmacogenetics. 2003;13:89–95.
Fanta S, Niemi M, Jönsson S, Karlsson MO, Holmberg C, Neuvonen PJ, et al. Pharmacogenetics of cyclosporine in children suggests an age-dependent influence of ABCB1 polymorphisms. Pharmacogenet Genomics. 2008;18:77–90.
García M, Macías RM, Cubero JJ, Benítez J, Caravaca F, Gervasini G. ABCB1 polymorphisms are associated with cyclosporine-induced nephrotoxicity and gingival hyperplasia in renal transplant recipients. Eur J Clin Pharmacol. 2013;69:385–93.
Qiu X-Y, Jiao Z, Zhang M, Zhong L-J, Liang H-Q, Ma C-L, et al. Association of MDR1, CYP3A4*18B, and CYP3A5*3 polymorphisms with cyclosporine pharmacokinetics in Chinese renal transplant recipients. Eur J Clin Pharmacol. 2008;64:1069–84.
Taegtmeyer AB, Breen JB, Smith J, Burke M, Leaver N, Pantelidis P, et al. ATP-binding cassette subfamily B member 1 polymorphisms do not determine cyclosporin exposure, acute rejection or nephrotoxicity after heart transplantation. Transplantation. 2010;89:75–82.
Wang Y, Wang C, Li J, Wang X, Zhu G, Chen X, et al. Effect of genetic polymorphisms of CYP3A5 and MDR1 on cyclosporine concentration during the early stage after renal transplantation in Chinese patients co-treated with diltiazem. Eur J Clin Pharmacol. 2009;65:239–47.
Yates CR, Zhang W, Song P, Li S, Gaber AO, Kotb M, et al. The effect of CYP3A5 and MDR1 polymorphic expression on cyclosporine oral disposition in renal transplant patients. J Clin Pharmacol. 2003;43:555–64.
Bouamar R, Hesselink DA, van Schaik RHN, Weimar W, Macphee IAM, de Fijter JW, et al. Polymorphisms in CYP3A5, CYP3A4, and ABCB1 are not associated with cyclosporine pharmacokinetics nor with cyclosporine clinical end points after renal transplantation. Ther Drug Monit. 2011;33:178–84.
Hesselink DA, van Gelder T, van Schaik RHN, Balk AHMM, van der Heiden IP, van Dam T, et al. Population pharmacokinetics of cyclosporine in kidney and heart transplant recipients and the influence of ethnicity and genetic polymorphisms in the MDR-1, CYP3A4, and CYP3A5 genes. Clin Pharmacol Ther. 2004;76:545–56.
Kuzuya T, Kobayashi T, Moriyama N, Nagasaka T, Yokoyama I, Uchida K, et al. Amlodipine, but not MDR1 polymorphisms, alters the pharmacokinetics of cyclosporine A in Japanese kidney transplant recipients. Transplantation. 2003;76:865–8.
Mai I, Störmer E, Goldammer M, Johne A, Krüger H, Budde K, et al. MDR1 haplotypes do not affect the steady-state pharmacokinetics of cyclosporine in renal transplant patients. J Clin Pharmacol. 2003;43:1101–7.
Min DI, Ellingrod VL, Marsh S, McLeod H. CYP3A5 polymorphism and the ethnic differences in cyclosporine pharmacokinetics in healthy subjects. Ther Drug Monit. 2004;26:524–8.
Press RR, Ploeger BA, den Hartigh J, van der Straaten T, van Pelt H, Danhof M, et al. Explaining variability in ciclosporin exposure in adult kidney transplant recipients. Eur J Clin Pharmacol. 2010;66:579–90.
Kim H-J, Im S-A, Keam B, Ham HS, Lee KH, Kim TY, et al. ABCB1 polymorphism as prognostic factor in breast cancer patients treated with docetaxel and doxorubicin neoadjuvant chemotherapy. Cancer Sci. 2015;106:86–93.
Lal S, Wong ZW, Sandanaraj E, Xiang X, Ang PCS, Lee EJD, et al. Influence of ABCB1 and ABCG2 polymorphisms on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008;99:816–23.
Voon PJ, Yap HL, Ma C-Y-T, Lu F, Wong ALA, Sapari NS, et al. Correlation of aldo-ketoreductase (AKR) 1C3 genetic variant with doxorubicin pharmacodynamics in Asian breast cancer patients. Br J Clin Pharmacol. 2013;75:1497–505.
Fukudo M, Ikemi Y, Togashi Y, Masago K, Kim YH, Mio T, et al. Population pharmacokinetics/pharmacodynamics of erlotinib and pharmacogenomic analysis of plasma and cerebrospinal fluid drug concentrations in Japanese patients with non-small cell lung cancer. Clin Pharmacokinet. 2013;52:593–609.
Kasuya K, Tsuchida A, Nagakawa Y, Suzuki Y, Suzuki M, Aoki T, et al. Prediction of a side effect and efficacy of adjuvant chemotherapy with gemcitabine for post operative patient of pancreatic cancer by a genetic polymorphism analysis. Hepatogastroenterology. 2012;59:1609–13.
Zhou Q, Sparreboom A, Tan E-H, Cheung Y-B, Lee A, Poon D, et al. Pharmacogenetic profiling across the irinotecan pathway in Asian patients with cancer. Br J Clin Pharmacol. 2005;59:415–24.
Gréen H, Söderkvist P, Rosenberg P, Horvath G, Peterson C. mdr-1 single nucleotide polymorphisms in ovarian cancer tissue: G2677T/A correlates with response to paclitaxel chemotherapy. Clin Cancer Res. 2006;12:854–9.
Gréen H, Söderkvist P, Rosenberg P, Horvath G, Peterson C. ABCB1 G1199A polymorphism and ovarian cancer response to paclitaxel. J Pharm Sci. 2008;97:2045–8.
Schaich M, Kestel L, Pfirrmann M, Robel K, Illmer T, Kramer M, et al. A MDR1 (ABCB1) gene single nucleotide polymorphism predicts outcome of temozolomide treatment in glioblastoma patients. Ann Oncol. 2009;20:175–81.
Guilhaumou R, Solas C, Bourgarel-Rey V, Quaranta S, Rome A, Simon N, et al. Impact of plasma and intracellular exposure and CYP3A4, CYP3A5, and ABCB1 genetic polymorphisms on vincristine-induced neurotoxicity. Cancer Chemother Pharmacol. 2011;68:1633–8.
Kang H-A, Cho H-Y, Lee Y-B. The effect of MDR1 G2677T/A polymorphism on pharmacokinetics of gabapentin in healthy Korean subjects. Arch Pharm Res. 2007;30:96–101.
Basic S, Hajnsek S, Bozina N, Filipcic I, Sporis D, Mislov D, et al. The influence of C3435T polymorphism of ABCB1 gene on penetration of phenobarbital across the blood–brain barrier in patients with generalized epilepsy. Seizure. 2008;17:524–30.
Hung C-C, Tai JJ, Lin C-J, Lee M-J, Liou H-H. Complex haplotypic effects of the ABCB1 gene on epilepsy treatment response. Pharmacogenomics. 2005;6:411–7.
Kwan P, Wong V, Ng PW, Lui CHT, Sin NC, Poon WS, et al. Gene-wide tagging study of association between ABCB1 polymorphisms and multidrug resistance in epilepsy in Han Chinese. Pharmacogenomics. 2009;10:723–32.
Karlsson L, Green H, Zackrisson AL, Bengtsson F, Jakobsen Falk I, Carlsson B, et al. ABCB1 gene polymorphisms are associated with fatal intoxications involving venlafaxine but not citalopram. Int J Legal Med. 2013;127:579–86.
Consoli G, Lastella M, Ciapparelli A, Catena Dell‘Osso M, Ciofi L, Guidotti E, et al. ABCB1 polymorphisms are associated with clozapine plasma levels in psychotic patients. Pharmacogenomics. 2009;10:1267–76.
Cho HY, Yoo HD, Lee YB. Influence of ABCB1 genetic polymorphisms on the pharmacokinetics of levosulpiride in healthy subjects. Neuroscience. 2010;169:378–87.
Bozina N, Kuzman MR, Medved V, Jovanovic N, Sertic J, Hotujac L. Associations between MDR1 gene polymorphisms and schizophrenia and therapeutic response to olanzapine in female schizophrenic patients. J Psychiatr Res. 2008;42:89–97.
Ghotbi R, Mannheimer B, Aklillu E, Suda A, Bertilsson L, Eliasson E, et al. Carriers of the UGT1A4 142T>G gene variant are predisposed to reduced olanzapine exposure—an impact similar to male gender or smoking in schizophrenic patients. Eur J Clin Pharmacol. 2010;66:465–74.
Lin Y-C, Ellingrod VL, Bishop JR, Miller DD. The relationship between P-glycoprotein (PGP) polymorphisms and response to olanzapine treatment in schizophrenia. Ther Drug Monit. 2006;28:668–72.
Kuzman MR, Medved V, Bozina N, Hotujac L, Sain I, Bilusic H. The influence of 5-HT(2C) and MDR1 genetic polymorphisms on antipsychotic-induced weight gain in female schizophrenic patients. Psychiatry Res. 2008;160:308–15.
Menu P, Gressier F, Verstuyft C, Hardy P, Becquemont L, Corruble E. Antidepressants and ABCB1 gene C3435T functional polymorphism: a naturalistic study. Neuropsychobiology. 2010;62:193–7.
Acknowledgments
This work was supported by the German Federal Ministry of Education and Research (Virtual Liver Network Grant No. 2318 0315755 and Grant No. 03 IS 2061C), Deutsche Forschungsgemeinschaft KFO 274 (Grant No. SCHW858/1-1), Robert Bosch Stiftung, Stuttgart, Germany, and a University of Tübingen—Faculty of Medicine PATE grant (Project No. F1315013).
SW, ES, HL, MS and ATN have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wolking, S., Schaeffeler, E., Lerche, H. et al. Impact of Genetic Polymorphisms of ABCB1 (MDR1, P-Glycoprotein) on Drug Disposition and Potential Clinical Implications: Update of the Literature. Clin Pharmacokinet 54, 709–735 (2015). https://doi.org/10.1007/s40262-015-0267-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0267-1