Abstract
Purpose
Fedratinib, an oral selective kinase inhibitor with activity against both wild type and mutationally activated Janus kinase 2, has been approved for the treatment of adult patients with intermediate-2 or high-risk myelofibrosis by the US Food and Drug Administration. In vitro studies indicated that fedratinib was an inhibitor of several cytochrome P450 (CYP) enzymes. The primary objective of this study was to evaluate the effects of repeated doses of fedratinib on the activity of CYP2D6, CYP2C19, and CYP3A4 in patients with solid tumors using a CYP probe cocktail.
Methods
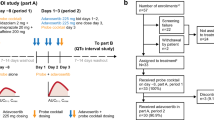
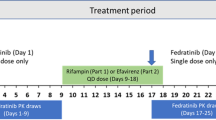
An open-label, one-sequence, two-period, two-treatment crossover study was conducted. Patients were administered a single oral dose cocktail of metoprolol (100 mg), omeprazole (20 mg), and midazolam (2 mg) used as probe substrates for CYP2D6, CYP2C19, and CYP3A4 enzyme activities, respectively, without fedratinib on Day -1 or with fedratinib on Day 15.
Results
Coadministration of 500 mg once-daily doses of fedratinib for 15 days increased the mean area under the plasma concentration–time curve from time zero to infinity following a single-dose cocktail containing metoprolol (CYP2D6 substrate), omeprazole (CYP2C19 substrate), and midazolam (CYP3A4 substrate) by 1.77-fold (90% confidence interval [CI] 1.27–2.47) for metoprolol, 2.82-fold (90% CI 2.26–3.53) for omeprazole, and 3.84-fold (90% CI 2.62–5.63) for midazolam, respectively. The mean plasma Day 14/Day 1 ratio of 4β-hydroxycholesterol, an endogenous biomarker of CYP3A4 activity, was 0.59 (90% CI 0.54-0.66), suggesting a net inhibition of CYP3A4 by fedratinib.
Conclusion
Fedratinib is a weak inhibitor of CYP2D6, and a moderate inhibitor of CYP2C19 and CYP3A4. These results serve as the basis for dose modifications of these CYP substrate drugs when co-administered with fedratinib.


Similar content being viewed by others
References
Vainchenker W, Constantinescu SN (2013) JAK/STAT signaling in hematological malignancies. Oncogene 32(21):2601–2613. https://doi.org/10.1038/onc.2012.347
Romano M, Sollazzo D, Trabanelli S, Barone M, Polverelli N, Perricone M et al (2017) Mutations in JAK2 and Calreticulin genes are associated with specific alterations of the immune system in myelofibrosis. Oncoimmunology 6(10):e1345402. https://doi.org/10.1080/2162402X.2017.1345402
U.S. Food and Drug Administration. INREBIC (fedratinib) label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf. Accessed Nov 12 2019
Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D et al (2015) Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol 1(5):643–651. https://doi.org/10.1001/jamaoncol.2015.1590
Harrison CN, Schaap N, Vannucchi AM, Kiladjian JJ, Tiu RV, Zachee P et al (2017) Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol 4(7):e317–e324. https://doi.org/10.1016/S2352-3026(17)30088-1
Tefferi A (2016) Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol 91(12):1262–1271. https://doi.org/10.1002/ajh.24592
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM et al (2011) Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 29(7):789–796. https://doi.org/10.1200/JCO.2010.32.8021
Zhang M, Xu CR, Shamiyeh E, Liu F, Yin JY, von Moltke LL et al (2014) A randomized, placebo-controlled study of the pharmacokinetics, pharmacodynamics, and tolerability of the oral JAK2 inhibitor fedratinib (SAR302503) in healthy volunteers. J Clin Pharmacol 54(4):415–421. https://doi.org/10.1002/jcph.218
Ogasawara K, Zhou S, Krishna G, Palmisano M, Li Y (2019) Population pharmacokinetics of fedratinib in patients with myelofibrosis, polycythemia vera, and essential thrombocythemia. Cancer Chemother Pharmacol 84(4):891–898. https://doi.org/10.1007/s00280-019-03929-9
Pardanani A, Tefferi A, Jamieson C, Gabrail NY, Lebedinsky C, Gao G et al (2015) A phase 2 randomized dose-ranging study of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J. 5:e335. https://doi.org/10.1038/bcj.2015.63
Zhang M, Xu C, Ma L, Shamiyeh E, Yin J, von Moltke LL et al (2015) Effect of food on the bioavailability and tolerability of the JAK2-selective inhibitor fedratinib (SAR302503): results from two phase I studies in healthy volunteers. Clin Pharmacol Drug Dev 4(4):315–321. https://doi.org/10.1002/cpdd.161
U.S. Food and Drug Administration. Clinical Drug Interaction Studies - Study Design, Data Analysis, and Clinical Implications, Guidance for Industry. 2017. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-study-design-data-analysis-and-clinical-implications-guidance. Accessed Nov 12 2019
U.S. Food and Drug Administration. In Vitro Metabolism- and Transporter-Mediated Drug-Drug Interaction Studies, Guidance for Industry. 2017. https://www.fda.gov/files/drugs/published/In-Vitro-Metabolism–and-Transporter–Mediated-Drug-Drug-Interaction-Studies-Guidance-for-Industry.pdf. Accessed Apr 2 2020
Turpault S, Brian W, Van Horn R, Santoni A, Poitiers F, Donazzolo Y et al (2009) Pharmacokinetic assessment of a five-probe cocktail for CYPs 1A2, 2C9, 2C19, 2D6 and 3A. Br J Clin Pharmacol 68(6):928–935. https://doi.org/10.1111/j.1365-2125.2009.03548.x
U.S. Food and Drug Administration. PRILOSEC (omeprazole) label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019810s102,022056s019lbl.pdf. Accessed Nov 12 2019
Bodin K, Bretillon L, Aden Y, Bertilsson L, Broome U, Einarsson C et al (2001) Antiepileptic drugs increase plasma levels of 4beta-hydroxycholesterol in humans: evidence for involvement of cytochrome p450 3A4. J Biol Chem 276(42):38685–38689. https://doi.org/10.1074/jbc.M105127200
Diczfalusy U, Nylen H, Elander P, Bertilsson L (2011) 4beta-Hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br J Clin Pharmacol 71(2):183–189. https://doi.org/10.1111/j.1365-2125.2010.03773.x
Zhou H, Tong Z, McLeod JF (2004) “Cocktail” approaches and strategies in drug development: valuable tool or flawed science? J Clin Pharmacol 44(2):120–134. https://doi.org/10.1177/0091270003261333
U.S. Food and Drug Administration. LOPRESSOR (metoprolol tartrate) label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/017963s068lbl.pdf. Accessed Nov 12 2019
Juif PE, Boehler M, Donazzolo Y, Bruderer S, Dingemanse J (2017) A pharmacokinetic drug-drug interaction study between selexipag and midazolam, a CYP3A4 substrate, in healthy male subjects. Eur J Clin Pharmacol 73(9):1121–1128. https://doi.org/10.1007/s00228-017-2282-7
Acknowledgements
The authors thank all study participants, their families and clinical study team members.
Funding
The clinical trial reported in this manuscript was sponsored by Sanofi, NJ, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KO., M.P, and G.K. are employees and hold equity ownership in Bristol Myers Squibb. P.M.L. is advisory board member from AbbVie, GenMab, Genentech, CytomX, Takeda, Cybrexa, Agenus, IQVIA, TRIGR, Pfizer, I-MAB, ImmunoMet, Black Diamond, Glaxo-Smith Kline, QED Therapeutics, AstraZeneca, EMD Serono, Shattuck, Astellas, Salarius, Silverback, MacroGenics; data safety monitoring board member from Agios, Five Prime, Halozyme, Tyme; member from imCORE Alliance (Roche-Genentech); and consultatnt of SOTIO. A.J.O. is advisory board member, with honoraria from: Array, BMS, Merck, Novartis and Pfizer. Trial funding (to institution) from: Adaptimmune; Alkermes; Astellas; BMS; Boston Biomedical; Checkmate Pharma; EMD Serono; Glenmark; Glyconex; GSK; Immunocore; Intensity Therapeutics; Kadmon; Kartos; Kura; Oncoceutics; OncoSec; Seattle Genetics; Sound Biologics; Spring Bank; Takeda; Targovax. O.R. does not have COI to disclose. C.X. and J.Y. are employees and hold equity ownership in Sanofi.
Ethical approval
The protocol and its amendments were submitted to local Ethics Committees, Institutional Review Boards for review and written approval. The protocol complied with recommendations of the 18th World Medical Assembly (Helsinki, 1964) and all applicable amendments with the laws and regulations, as well as any applicable guidelines of the United States, where the study was conducted. Informed consent was obtained at screening, prior to the conduct of any study-related procedures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ogasawara, K., LoRusso, P.M., Olszanski, A.J. et al. Assessment of effects of repeated oral doses of fedratinib on inhibition of cytochrome P450 activities in patients with solid tumors using a cocktail approach. Cancer Chemother Pharmacol 86, 87–95 (2020). https://doi.org/10.1007/s00280-020-04102-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04102-3