Abstract
Purpose
EC0305 represents a folate-tubulysin B construct capable of specifically eradicating folate receptor (FR)-positive subcutaneous tumors from mice (Leamon et al., Cancer Res 68:9839–9844, 8). Herein we report on the use of multiple polar carbohydrate segments (e.g. 1-amino-1-deoxy-glucitolyl-γ-glutamate) placed in-between the folate and tubulysin B moieties of EC0305 creating a new conjugate, herein referred to as EC0531, with more desirable biological properties.
Methods
The synthesis of EC0531 and its tritium-labeled counterpart are described. EC0531’s affinity for FR binding and specific cytotoxic activity was assessed using standard in vitro assays. Human tumor xenografts were used to directly compare EC0305 and EC0531’s antitumor activity. Finally, bile duct cannulated, female Sprague–Dawley rats were used to compare hepatobiliary clearance of these two targeted chemotherapeutic agents.
Results
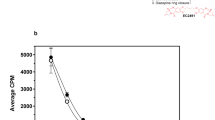
EC0531 tightly binds to the FR with an affinity about half that of folic acid. It was found to specifically inhibit the growth of FR+ cells (IC50 of ~2 nM) in a dose-dependent manner. Using 3H-labeled compounds, more than a 12-fold higher amount of tubulysin was measured in a FR + human tumor xenograft compared to the unconjugated drug, a finding that explains, in part, why EC0531 displays curative activity, whereas the unconjugated tubulysin agent is essentially inactive. EC0531 was found to produce greater FR-specific anti-tumor activity at lower dose levels than EC0305; furthermore, EC0531’s maximum tolerated dose level was significantly higher than that of EC0305, likely because EC0531’s saccharopeptidic-based spacer allows for ~sixfold reduction in hepatic clearance.
Conclusions
These data provide additional evidence that the therapeutic range of targeted small-molecule drug conjugates can be favorably increased using molecular spacers constructed with 1-amino-1-deoxy-glucitolyl-γ-glutamate residues.





Similar content being viewed by others
References
Leamon CP, Low PS (1991) Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc Natl Acad Sci USA 88:5572–5576
Leamon CP (2008) Folate-targeted drug strategies for the treatment of cancer. Curr Opin Investig Drugs 9(12):1277–1286
Muller C, Schibli R (2013) Prospects in folate receptor-targeted radionuclide therapy. Front Oncol 3:249
Zwicke GL, Mansoori GA, Jeffery CJ (2012) Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev 3:1–11
Vlahov IR, Wang Y, Kleindl PJ, Leamon CP (2008) Design and regioselective synthesis of a new generation of targeted chemotherapeutics. Part II: folic acid conjugates of tubulysins and their hydrazides. Bioorg Med Chem Lett 18(16):4558–4561
Sasse F, Steinmetz H, Heil J, Hofle G, Reichenbach H (2000) Tubulysins, new cytostatic peptides from myxobacteria acting on microtubuli. Production, isolation, physico-chemical and biological properties. J Antibiot (Tokyo) 53(9):879–885
Steinmetz H, Glaser N, Herdtweck E, Sasse F, Reichenbach H, Hofle G (2004) Isolation, crystal and solution structure determination, and biosynthesis of tubulysins—powerful inhibitors of tubulin polymerization from myxobacteria. Angew Chem Int Ed Engl 43(37):4888–4892
Leamon CP, Reddy JA, Vetzel M, Dorton R, Westrick E, Parker N, Wang Y, Vlahov I (2008) Folate targeting enables durable and specific antitumor responses from a therapeutically null tubulysin B analogue. Cancer Res 68(23):9839–9844
Reddy JA, Dorton R, Dawson A, Vetzel M, Parker N, Nicoson JS, Westrick E, Klein PJ, Wang Y, Vlahov IR, Leamon CP (2009) In vivo structural activity and optimization studies of folate-tubulysin conjugates. Mol Pharm 6(5):1518–1525
Leamon CP, Reddy JA, Klein PJ, Vlahov IR, Dorton R, Bloomfield A, Nelson M, Westrick E, Parker N, Bruna K, Vetzel M, Gehrke M, Nicoson JS, Messmann RA, LoRusso PM, Sausville EA (2011) Reducing undesirable hepatic clearance of a tumor-targeted vinca alkaloid via novel saccharopeptidic modifications. J Pharmacol Exp Ther 336(2):336–343
Xu L, Vlahov IR, Leamon CP, Santhapuram HKR, Li CH (2005) Synthesis, purification, and uses of pteroic acid and derivatives and conjugates thereof. Patent #8,044,200 B2; issued 10-25-11, filed March 14, 2006
Lu Y, Stinnette TW, Westrick E, Klein PJ, Gehrke MA, Cross VA, Vlahov IR, Low PS, Leamon CP (2011) Treatment of experimental adjuvant arthritis with a novel folate receptor-targeted folic acid-aminopterin conjugate. Arthritis Res Ther 13(2):R56
Vlahov IR, Santhapuram HKR, You F, Wang Y, Kleindl PJ, Hahn SJ, Vaughn JF, Reno DS, Leamon CP (2010) Carbohydrate-based synthetic approach to control toxicity profiles of folate-drug conjugates. J Org Chem 75(11):3685–3691
Vlahov IR, Santhapuram HK, Kleindl PJ, Howard SJ, Stanford KM, Leamon CP (2006) Design and regioselective synthesis of a new generation of targeted chemotherapeutics. Part 1: EC145, a folic acid conjugate of desacetylvinblastine monohydrazide. Bioorg Med Chem Lett 16(19):5093–5096
Leamon CP, You F, Santhapuram HK, Fan M, Vlahov IR (2009) Properties influencing the relative binding affinity of pteroate derivatives and drug conjugates thereof to the folate receptor. Pharm Res 26(6):1315–1323
Mathias CJ, Wang S, Lee RJ, Waters DJ, Low PS, Green MA (1996) Tumor-selective radiopharmaceutical targeting via receptor-mediated endocytosis of Gallium-67-deferoxamine-folate. J Nuc Med 37(6):1003–1008
Leamon CP, Reddy JA, Dorton R, Bloomfield A, Emsweller K, Parker N, Westrick E (2008) Impact of high and low folate diets on tissue folate receptor levels and antitumor responses toward folate-drug conjugates. J Pharmacol Exp Ther 327(3):918–925
Leamon CP, Reddy JA (2004) Folate-targeted chemotherapy. Adv Drug Deliv Rev 56(8):1127–1141
Paulos CM, Reddy JA, Leamon CP, Turk MJ, Low PS (2004) Ligand binding and kinetics of folate receptor recycling in vivo: impact on receptor-mediated drug delivery. Mol Pharmacol 66(6):1406–1414
Leamon CP, Reddy JA, Vlahov IR, Westrick E, Parker N, Nicoson JS, Vetzel M (2007) Comparative preclinical activity of the folate-targeted Vinca alkaloid conjugates EC140 and EC145. Int J Cancer 121(7):1585–1592
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical compliance
All in vivo studies were performed at Purdue University facilities in accordance with the American Accreditation Association of Laboratory Animal Care guidelines. All of the authors are or had been full time employees and stock holders of Endocyte, Inc. at the time of data collection and final analysis.
Rights and permissions
About this article
Cite this article
Leamon, C.P., Reddy, J.A., Vlahov, I.R. et al. Enhancing the therapeutic range of a targeted small-molecule tubulysin conjugate for folate receptor-based cancer therapy. Cancer Chemother Pharmacol 79, 1151–1160 (2017). https://doi.org/10.1007/s00280-017-3311-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3311-z