Abstract
Purpose
Addition of either nab-paclitaxel or erlotinib to gemcitabine to treat advanced pancreatic cancer has demonstrated overall survival benefit. This study was conducted to evaluate the tolerability and safety of combining all three drugs and assess preliminary evidence of efficacy.
Methods
In this open-label, phase 1b study, patients with previously untreated, advanced pancreatic cancer were treated in 28-day cycles with intravenous gemcitabine/nab-paclitaxel on days 1, 8, and 15, and once daily oral erlotinib. A standard “3 + 3” design was used. Dose level 1 (DL1) for gemcitabine (mg/m2)/nab-paclitaxel (mg/m2)/erlotinib (mg) was 1000/125/100, respectively, with de-escalation to DL−1 (1000/100/100), DL−2b (1000/75/100), and DL−3 (1000/75/75). The maximum tolerated dose (MTD) was defined by occurrence of dose-limiting toxicity (DLT) in ≤1 of six patients within the first cycle. Efficacy was assessed with CT scans performed at two-cycle intervals.
Results
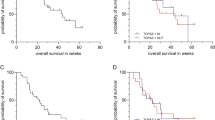
Nineteen patients were enrolled. DLTs occurred in two patients at DL1, three patients at DL−1, two patients at DL−2b, and one patient at DL−3. The MTD for the combination of gemcitabine/nab-paclitaxel/erlotinib was DL−3 (1000/75/75). In analyses of efficacy among 14 evaluable patients, partial responses were observed in four of six patients at DL1, one of two patients at DL−2b, and two of six patients at DL−3.
Conclusion
The addition of erlotinib to gemcitabine and nab-paclitaxel is not tolerable at standard single-agent dosing of all drugs. However, significant clinical activity was noted, even at DL−3. Further study of the combination will need to incorporate reduced dosing.

Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE et al (2011) Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 29:4548–4554
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Krishna K, Blazer MA, Wei L, Ahn DH, Wu CS, Ciombor KK et al (2015) Modified gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer (MPC): a single-institution experience. J Clin Oncol 33(3 suppl):366
Acknowledgments
The authors thank the patients and their families who participated in the study. Medical writing assistance was provided by Tara N. Miller, Ph.D., and Ed Parr, Ph.D., of Envision Scientific Solutions, which was funded by Astellas.
Funding
This study was funded by Astellas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Steven Cohen has received personal fees from Celgene Corporation. Jordan Berlin received institutional grants from Astellas related to the study and has received personal fees from Celgene. Julie Horan was an employee of OSI Pharmaceuticals during the conduct of the study. Patricia Catalano is an employee of Novella, who were contracted to perform services related to the study. Angela Davies was an employee of OSI Pharmaceuticals and Astellas during the conduct of the study. Bert O’Neil, Patricia Ames, Marti McKinley, Colin Weekes, and Lawrence Leichman have no conflicts to report.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Dr. Davies was an employee of Astellas when the study was conducted.
Rights and permissions
About this article
Cite this article
Cohen, S.J., O’Neil, B.H., Berlin, J. et al. A phase 1b study of erlotinib in combination with gemcitabine and nab-paclitaxel in patients with previously untreated advanced pancreatic cancer: an Academic Oncology GI Cancer Consortium study. Cancer Chemother Pharmacol 77, 693–701 (2016). https://doi.org/10.1007/s00280-016-2981-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-2981-2