Abstract
Background
TAS-102 is an oral fluoropyrimidine prodrug composed of trifluridine (FTD) and tipiracil hydrochloride (TPI) in a 1:0.5 ratio. FTD is a thymidine analog, and it is degraded by thymidine phosphorylase (TP) to the inactive trifluoromethyluracil (FTY) metabolite. TPI inhibits degradation of FTD by TP, increasing systemic exposure to FTD.
Methods
Patients with advanced solid tumors (6 M/2 F; median age 58 years; PS 0–1) were enrolled on this study. Patients in group A (N = 4) received 60 mg TAS-102 with 200 nCi [14C]-FTD, while patients in group B (N = 4) received 60 mg TAS-102 with 1000 nCi [14C]-TPI orally. Plasma, blood, urine, feces, and expired air (group A only) were collected up to 168 h and were analyzed for 14C by accelerator mass spectrometry and analytes by LC–MS/MS.
Results
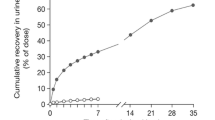
FTD: 59.8 % of the 14C dose was recovered: 54.8 % in urine mostly as FTY and FTD glucuronide isomers. The extractable radioactivity in the pooled plasma consisted of 52.7 % FTD and 33.2 % FTY. TPI: 76.8 % of the 14C dose was recovered: 27.0 % in urine mostly as TPI and 49.7 % in feces. The extractable radioactivity in the pooled plasma consisted of 53.1 % TPI and 30.9 % 6-HMU, the major metabolite of TPI.
Conclusion
Absorbed 14C-FTD was metabolized and mostly excreted in urine. The majority of 14C-TPI was recovered in feces, and the majority of absorbed TPI was excreted in urine. The current data with the ongoing hepatic and renal dysfunction studies will provide an enhanced understanding of the TAS-102 elimination profile.







Similar content being viewed by others
References
Tanaka N et al (2014) Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep 32(6):2319–2326
Temmink OH et al (2007) Therapeutic potential of the dual-targeted TAS-102 formulation in the treatment of gastrointestinal malignancies. Cancer Sci 98(6):779–789
Emura T et al (2004) A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. Int J Mol Med 13(4):545–549
Emura T et al (2004) A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol 25(3):571–578
Emura T et al (2005) Potentiation of the antitumor activity of alpha, alpha, alpha-trifluorothymidine by the co-administration of an inhibitor of thymidine phosphorylase at a suitable molar ratio in vivo. Int J Oncol 27(2):449–455
Overman MJ et al (2008) Phase I clinical study of three times a day oral administration of TAS-102 in patients with solid tumors. Cancer Investig 26(8):794–799
Overman MJ et al (2008) Phase 1 study of TAS-102 administered once daily on a 5-day-per-week schedule in patients with solid tumors. Investig New Drugs 26(5):445–454
Hong DS et al (2006) Phase I study to determine the safety and pharmacokinetics of oral administration of TAS-102 in patients with solid tumors. Cancer 107(6):1383–1390
Yoshino T et al (2012) TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 13(10):993–1001
Mayer RJ et al (2015) Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372(20):1909–1919
Kotani D, Fukuoka S, Yoshino T (2015) Efficacy of TAS-102. Gan To Kagaku Ryoho 42(1):1–5
Beumer JH et al (2007) Human mass balance study of the novel anticancer agent ixabepilone using accelerator mass spectrometry. Investig New Drugs 25(4):327–334
Graham RA et al (2011) A single dose mass balance study of the Hedgehog pathway inhibitor vismodegib (GDC-0449) in humans using accelerator mass spectrometry. Drug Metab Dispos 39(8):1460–1467
U.S. Department of Health and Human Services Food and Drug Administration (2001) Guidance for industry-bioanalytical method validation. U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation; and Research Center for Veterinary Medicine
Hamilton RA, Garnett WR, Kline BJ (1981) Determination of mean valproic acid serum level by assay of a single pooled sample. Clin Pharmacol Ther 29(3):408–413
Doi T et al (2012) Phase I study of TAS-102 treatment in Japanese patients with advanced solid tumours. Br J Cancer 107(3):429–434
Khalili P et al (2002) Pharmacokinetics and metabolism of the novel synthetic C-nucleoside, 1-(2-deoxy-beta-d-ribofuranosyl)-2,4-difluoro-5-iodobenzene: a potential mimic of 5-iodo-2′-deoxyuridine. Biopharm Drug Dispos 23(3):105–113
Zhou L et al (2010) Disposition of [1′-(14)C]stavudine after oral administration to humans. Drug Metab Dispos 38(4):655–666
Desmoulin F et al (2007) A glucuronidation pathway of capecitabine occurs in rats but not in mice and humans. Drug Metab Lett 1(2):101–107
Desmoulin F et al (2002) Metabolism of capecitabine, an oral fluorouracil prodrug: (19)F NMR studies in animal models and human urine. Drug Metab Dispos 30(11):1221–1229
Nicolas F et al (1995) Comparative metabolism of 3′-azido-3′-deoxythymidine in cultured hepatocytes from rats, dogs, monkeys, and humans. Drug Metab Dispos 23(3):308–313
Good SS et al (1990) Isolation and characterization of an ether glucuronide of zidovudine, a major metabolite in monkeys and humans. Drug Metab Dispos 18(3):321–326
Dexter DL et al (1972) The clinical pharmacology of 5-trifluoromethyl-2′-deoxyuridine. Cancer Res 32(2):247–253
Shields AF et al (1996) Analysis of 2-carbon-11-thymidine blood metabolites in PET imaging. J Nucl Med 37(2):290–296
Beumer JH, Beijnen JH, Schellens JH (2006) Mass balance studies, with a focus on anticancer drugs. Clin Pharmacokinet 45(1):33–58
Ghoos Y et al (1988) Measurement of 13C-glucose oxidation rate using mass spectrometric determination of the CO2: Ar ratio and spirometry. Biomed Environ Mass Spectrom 15(8):447–451
Rogers WI et al (1969) The fate of 5-trifluoromethyl-2′-deoxyuridine in monkeys, dogs, mice, and tumor-bearing mice. Cancer Res 29(4):953–961
Heidelberger C, Boohar J, Kampschroer B (1965) Fluorinated pyrimidines. Xxiv. In vivo metabolism of 5-trifluoromethyluracil-2-C-14 and 5-trifluoromethyl-2′-deoxyuridine-2-C-14. Cancer Res 25:377–381
Andersen JT et al (2014) Extending serum half-life of albumin by engineering neonatal Fc receptor (FcRn) binding. J Biol Chem 289(19):13492–13502
Hammond TG et al (2014) Mass spectrometric characterization of circulating covalent protein adducts derived from a drug acyl glucuronide metabolite: multiple albumin adductions in diclofenac patients. J Pharmacol Exp Ther 350(2):387–402
Meng X et al (2013) Detection of drug bioactivation in vivo: mechanism of nevirapine–albumin conjugate formation in patients. Chem Res Toxicol 26(4):575–583
Ariza A et al (2012) Protein haptenation by amoxicillin: high resolution mass spectrometry analysis and identification of target proteins in serum. J Proteomics 77:504–520
Wang J et al (2010) Characterization of HKI-272 covalent binding to human serum albumin. Drug Metab Dispos 38(7):1083–1093
Shipkova M et al (2002) Pharmacokinetics and protein adduct formation of the pharmacologically active acyl glucuronide metabolite of mycophenolic acid in pediatric renal transplant recipients. Ther Drug Monit 24(3):390–399
Wang M, Dickinson RG (2000) Bile duct ligation promotes covalent drug-protein adduct formation in plasma but not in liver of rats given zomepirac. Life Sci 68(5):525–537
Georges H et al (1999) Glycation of human serum albumin by acylglucuronides of nonsteroidal anti-inflammatory drugs of the series of phenylpropionates. Life Sci 65(12):PL151-6
Benet LZ et al (1993) Predictability of the covalent binding of acidic drugs in man. Life Sci 53(8):PL141-6
Klaassen CD (ed) (2008) Casarett and Doull’s toxicology: the basic science of Poisons, 7th edn. McGraw-Hill, New York, p 1309
Acknowledgments
We thank the patients and their family members for their dedication and commitment to this clinical study. We thank the nursing staff of the University of Pittsburgh Clinical Translational Research Center for their invaluable assistance. This work was supported by Taiho Oncology, Inc., and NIH/NCRR/CTSA Grant UL1 RR024153. This project used the UPCI Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and was supported in part by award P30CA047904. We would like to thank the following individuals and organizations for their input in the various stages of this project: Manuel Aivado, Brian Kiesel, Milind Narurkar, Mark Seymour, Dennis Swanson, Tracy Topp, Erin Sternberg, and JCL Bioassay USA Inc. The authors were responsible for all content and editorial decisions and received no honoraria related to the development of this publication. All authors contributed to the research, writing, and reviewing of all drafts of this publication, and all authors approved the final draft prior to submission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.J., Seraj, J., Yoshida, K. et al. Human mass balance study of TAS-102 using 14C analyzed by accelerator mass spectrometry. Cancer Chemother Pharmacol 77, 515–526 (2016). https://doi.org/10.1007/s00280-016-2965-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-2965-2