Summary
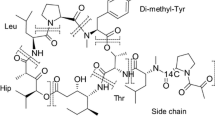
Plitidepsin (Aplidin®) is a marine-derived anticancer compound currently investigated in phase III clinical trials. This article describes the distribution, metabolism and excretion of this novel agent and it mainly aims to identify the major routes of elimination. Six subjects were enrolled in a mass balance study during which radiolabelled plitidepsin was administered as a 3-h intravenous infusion. Blood samples were taken and urine and faeces were collected. Total radioactivity (TRA) analysis using Liquid Scintillation Counting (LSC) was done to determine the amount of radioactivity excreted from the body and plitidepsin concentrations in whole blood, plasma and urine were determined by validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays. In total, a mean of 77.4% of the administered radioactivity was excreted over a time period of 20 days, of which 71.3% was recovered in faeces and 6.1% was found in urine. The majority excreted in urine was accounted for by unchanged plitidepsin, with only 1.5% of the total administered dose explained by metabolites in urine. Faeces, on the other hand contained low levels of parent compound, which means that most of the TRA excreted in faeces was accounted for by metabolites. TRA levels were 3.7 times higher in whole blood compared to plasma. Plitidepsin was widely distributed and plasma clearance was low. This study shows that red blood cells are a major distribution compartment and that the biliary route is the main route of total radioactivity excretion.




Similar content being viewed by others
References
Losada A, Muñoz-Alonso MJ, García C et al (2016) Translation elongation factor eEF1A2 is a novel anticancer target for the marine natural product plitidepsin. Sci Rep April:1–15. doi:10.1038/srep35100
Oliveira H, Thevenot J, Garanger E et al (2014) Nano-encapsulation of plitidepsin: in vivo pharmacokinetics, biodistribution, and efficacy in a renal xenograft tumor model. Pharm Res 31:983–991. doi:10.1007/s11095-013-1220-3
Danu A, Willekens C, Ribrag V (2013) Plitidepsin: an orphan drug. Expert Opin Orphan Drugs 1:569–580. doi:10.1517/21678707.2013.808995
Plummer R, Lorigan P, Brown E et al (2013) Phase I-II study of plitidepsin and dacarbazine as first-line therapy for advanced melanoma. Br J Cancer 109:1451–1459. doi:10.1038/bjc.2013.477
Ribrag V, Caballero D, Fermé C et al (2013) Multicenter phase II study of plitidepsin in patients with relapsed/refractory non-Hodgkin’s lymphoma. Haematologica 98:357–563. doi:10.3324/haematol.2012.069757
Nalda-Molina R, Valenzuela B, Ramon-Lopez A et al (2009) Population pharmacokinetics meta-analysis of plitidepsin (Aplidin) in cancer subjects. Cancer Chemother Pharmacol 64:97–108. doi:10.1007/s00280-008-0841-4
Kitagaki J, Shi G, Miyauchi S et al (2015) Cyclic depsipeptides as potential cancer therapeutics. Anti-Cancer Drugs 26:259–271. doi:10.1097/CAD.0000000000000183
Geoerger B, Estlin EJ, Aerts I et al (2012) A phase I and pharmacokinetic study of plitidepsin in children with advanced solid tumours: an innovative therapies for children with cancer (ITCC) study. Eur J Cancer 48:289–296. doi:10.1016/j.ejca.2011.10.036
Morande PE, Zanetti SR, Borge M et al (2012) The cytotoxic activity of Aplidin in chronic lymphocytic leukemia (CLL) is mediated by a direct effect on leukemic cells and an indirect effect on monocyte-derived cells. Investig New Drugs 30:1830–1840. doi:10.1007/s10637-011-9740-3
Mateos MV, Cibeira MT, Richardson PG et al (2010) Phase II clinical and pharmacokinetic study of plitidepsin 3-hour infusion every two weeks alone or with dexamethasone in relapsed and refractory multiple myeloma. Clin Cancer Res 16:3260–3269. doi:10.1158/1078-0432.CCR-10-0469
Eisen T, Thomas J, Miller WH Jr et al (2009) Phase II study of biweekly plitidepsin as second-line therapy in patients with advanced malignant melanoma. Melanoma Res 19:185–192. doi:10.1097/CMR.0b013e32832bbde6
Brandon EFA, Sparidans RW, van Ooijen RD et al (2006) In vitro characterization of the human biotransformation pathways of aplidine, a novel marine anti-cancer drug. Investig New Drugs 25:9–19. doi:10.1007/s10637-006-7589-7
He K, Iyer KR, Hayes RN et al (1998) Inactivation of cytochrome P450 3A4 by bergamottin, a component of grapefruit juice. Chem Res Toxicol 11:252–259. doi:10.1021/tx970192k
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Nijenhuis CM, Schellens JHM, Beijnen JH (2016) Regulatory aspects of human radiolabeled mass balance studies in oncology: concise review. Drug Metab Rev 48:266–280. doi:10.1080/03602532.2016.1181081
Izquierdo MA, Bowman A, García M et al (2008) Phase I clinical and pharmacokinetic study of plitidepsin as a 1-hour weekly intravenous infusion in patients with advanced solid tumors. Clin Cancer Res 14:3105–3112. doi:10.1158/1078-0432.CCR-07-1652
Faivre S, Chièze S, Delbaldo C et al (2005) Phase I and pharmacokinetic study of aplidine, a new marine cyclodepsipeptide in patients with advanced malignancies. J Clin Oncol 23:7871–7880. doi:10.1200/JCO.2005.09.357
Maroun JA, Belanger K, Seymour L et al (2006) Phase I study of Aplidine in a daily × 5 one-hour infusion every 3 weeks in patients with solid tumors refractory to standard therapy. A National Cancer Institute of Canada clinical trials group study: NCIC CTG IND 115. Ann Oncol 17:1371–1378. doi:10.1093/annonc/mdl165
Perez-Ruixo JJ, Zannikos P, Hirankarn S et al (2007) Population pharmacokinetic meta-analysis of trabectedin (ET-743, Yondelis) in cancer patients. Clin Pharmacokinet 46:867–884
Penner N, Klunk LJ, Prakash C (2009) Human radiolabaled mass balance studies: objectives, utilities and limitations. Biopharm Drug Dispos 30:185–203. doi:10.1002/bdd.661
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SF, SM, IG are currently employees, and BM-L was a former employee at Pharma Mar, S.A. LA, HR, MMT, NV, AHMVS, JHMS and JHB are employed at the Netherlands Cancer Institute.
Funding
This study was funded by Pharma Mar, S.A.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the participating institution and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
van Andel, L., Fudio, S., Rosing, H. et al. Pharmacokinetics and excretion of 14C–Plitidepsin in patients with advanced cancer. Invest New Drugs 35, 589–598 (2017). https://doi.org/10.1007/s10637-017-0432-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0432-5