Abstract
Aim
Hydrophilic drugs particularly those with low plasma protein binding may accumulate in third-space fluid in the body. Cytotoxic drugs like methotrexate (MTX) cause damage in the tissue, and evacuation of the third-space fluid in pleura is strongly recommended before new dosing. Pemetrexed (PEM) is a multi-targeted antifolate similar to MTX approved for the treatment for malignant pleural mesothelioma and non-small cell lung cancer. Current recommendations for patients receiving treatment with PEM prescribe draining of the pleural fluid. This is based upon the recommendations for MTX and not directly to any specific findings relating to PEM. The recommendations are the same because PEM is an analogue of MTX; the molecular structures and pharmacokinetic parameters are similar. However, since draining the pleural fluid is painful and cancer patient are particularly susceptible to infection subsequently, it is relevant to examine the recommendations for PEM explicitly.
Method
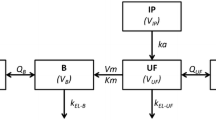
Eight patients treated with a 500 mg/m2 PEM combined with platinum salt were examined. Plasma samples were first collected in relation to the start of PEM infusion. Thereafter, plasma and pleura samples were taken at various times after drug infusion from each patient; in two patients, sampling was done twice but on different occasions. The quantitative determination of PEM was performed with reversed-phase high-performance liquid chromatography, and sample preparation was performed using protein precipitation with perchloric acid. Pharmacokinetic analysis was performed using a non-compartment method as well a two-compartment model.
Results
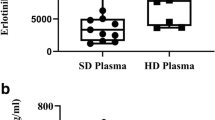
The results were calculated from 10 samples taken from eight patients, where data from one patient point were excluded as the patient had impaired renal function, and three samples were reported as below limit of quantification. The plasma PEM pharmacokinetics calculated showed an elimination half-life (t ½ elimination) of 3.2 h and distribution half-life (t ½-distribution) of 6 min. Clearance (CL) was 5.1 L/h, central volume of distribution (V central) 23.2 L and peripheral volume distribution (V peripheral) 10.6 L, and the area under the curve was 186 μg h/mL. Using non-compartment methods, an elimination half-life of 3.1 h and an apparent CL of 3.2 L/h were measured, whereas an apparent steady-state volume became 14.2 L. The pleura concentrations were only half of simultaneous plasma concentrations, and elimination half-life was 3.15 h.
Conclusion
Pemetrexed is not likely to accumulate in the pleural fluid, and evacuation of fluid might not be necessary. Further investigation is needed to recommend no drainage of the fluid, i.e., in patients with renal impairment.

Similar content being viewed by others
References
Frei E III, Jaffe N, Tattersall MH, Pitman S, Parker L (1975) New approaches to cancer chemotherapy with methotrexate. New Engl J Med 292:846
Evans WE, Pratt CB (1978) Effect of pleural effusion on high-dose methotrexate kinetics. Clin Pharmacol Ther 23(1):68–72
Cotes JE (2006) Lung function: physiology, measurement and application in medicine, Chapter 1 and 2, 6th edn. Blackwell publishing, Australia. ISBN 978-0-632-06493-9
Thomas JT, Musani AI (2013) Malignant pleural effusions: a review. Clin Chest Med 34:459–471
Hyeon Y (2011) Management of pleural effusion, empyema and lung abscess. Semin Intervent Radiol 28(1):75–86
Light R, Lee GY (2008) Textbook of pleural diseases, 2nd edn. Hodder Education, London
Pass HI, Carbone DP, Johnson DH, Minna JD et al (2010) Principles and practice of lung cancer, 4th edn. Lippincott Williams & Wilkins, USA. ISBN 978-0-7817-7365-2
Zarogoulidis K, Zarogoulidis P, Darwiche K, Tsakiridis K et al (2013) Malignant pleural effusion and algorithm management. J Thorac Dis 5(54):S413–S419
Putnam JB (2002) Malignant pleural effusions. Surg Clin North Am 82:867–883
Hung TL, Chen FF, Liu JM, Lai WW et al (2003) Clinical evaluation of HER-2/neu protein in malignant pleural effusion-associated lung adenocarcinoma and as tumor marker in pleural effusion diagnosis. Clin Cancer Res 9:2605–2612
Gonlugur TE, Gonlugur U (2008) Transudates in malignancy: still a role for pleural fluid. Ann Acad Med Singapore 37(9):760–763
Thomas R, Lee G (2013) Causes and management of common benign pleural effusions. Thorac Surg Clin 23:25–42
Clementsen PS (2001) Pleuracentese. Ugeskr Læger 163:2639–2640
Light RW, Hamm H (1997) Malignant pleural effusion: would the real cause please stand up? Eur Respir J 10:1701–1702
Kaifi JT, Toth JW, Gusani NJ, Kimichi ET et al (2012) Multidisciplinary management of malignant pleural effusion. J Surg Oncol 105:731–738
Rinaldi DA, Burris HA, Dorr FA, Woodworth JG et al (1995) Initial phase I evaluation of the novel thymidylate synthase inhibitor, LY231514, using the modified continual reassessment method for dose escalation. J Clin Oncol 13(11):2842–2850
Guidance for Industry—Bioanalytical Method Validation (2010) U S Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research 2001. pp 1–22
Altman D, Bland MJ (2005) Standard deviations and standard errors. Statistics note. BMJ 331(7521):903
Rosenbaum SE (2011) Basic pharmacokinetic and pharmacodynamic: an integrated textbook and computer simulations. Wiley, New Jersey. ISBN 978-0-470-56906-1
Rowland M, Tozer TN (2011) Clinical pharmacokinetics and pharmacodynamics: concepts and applications, 4th edn. Williams & Wilkins, Lippincott
Guidance for Industry (1998) Pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. http://www.fda.gov/downloads/Drugs/Guidances/UCM204959.pdf
Rinaldi DA, Kuhn JG, Burris HA, Dorr FA et al (1999) A phase I evaluation of multi-targeted antifolate (MTA, LY231514), administered every 21 days, utilizing the modified continual reassessment method for dose escalation. Cancer Chemother Pharmacol 44:372–380
McDonald AC, Vasey PA, Adams L et al (1998) A phase I and pharmacokinetic study of LY231514, the multitargeted antifolate. Clin Cancer Res 4:605–610
Quellet D, Periclou AP, Johnson RD, Woodworth JR et al (2000) Population pharmacokinetics of pemetrexed disodium (ALIMTA) in patients with cancer. Cancer Chemother Pharmacol 46:227–234
Dickgreber NJ, Fink TH, Latz JE, Hossain AM et al (2009) Phase I and pharmacokinetic study of pemetrexed plus cisplatin in chemonaive patients with locally advanced or metastatic malignant pleural mesothelioma or non-small cell lung cancer. Clin Cancer Res 15:382–389
Dickgreber NJ, Sorensen JB, Paz-Ares LG, Schytte TK et al (2010) Pemetrexed safety and pharmacokinetics in patients with third-space fluid. Clin Cancer Res 16(10):2872–2880
Nakagawa K, Kudoh S, Matsui K, Negoro S et al (2006) A phase I study of pemetrexed (LY231514) supplemented with folate and vitamin B12 in Japanese patients with solid tumours. Br J Cancer 95:677–682
Latz JE, Chaudhary A, Ghosh A, Johnson RD (2006) Population pharmacokinetic analysis of ten phase II clinical trials of pemetrexed in cancer patients. Cancer Chemother Pharmacol 57:401–411
Hughes A, Calvert P, Azzabi A, Plummer R et al (2002) Phase I clinical and pharmacokinetic study of pemetrexed and carboplatin in patients with malignant pleural mesothelioma. J Clin Oncol 20(16):3533–3544
Li J, Gwilt P (2002) The effect of malignant effusions on methotrexate disposition. Cancer Chemother Pharmacol 50:373–382
Mita AC, Sweeney CJ, Baker SD, Goetz A et al (2006) Phase I and pharmacokinetic study of pemetrexed administered every 3 weeks to advanced cancer patients with normal and impaired renal function. J Clin Oncol 24(4):552–562
Undevia SD, Gomez-Abuin G, Ratain MJ (2005) Pharmacokinetic variability of anticancer agents. Nat Rev Cancer 5:447–458
Huffman DH (1973) Pharmacokinetics of methotrexate. Clin Pharmacol Ther 14:572–579
Chabner BA, Young RC (1973) Threshold methotrexate concentration for in vivo inhibition of DNA synthesis in normal and tumorous target tissues. J Clin Invest 52:1804–1811
Beal SL (2001) Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28(5):481–504
Bergstrand M, Karlsson MO (2009) Handling data below the limit of quantification in mixed effect models. AAPS J 11(2):371–380
Duval V, Karlsson MO (2002) Impact of omission or replacement of data below the limit of quantification on parameter estimates in a two-compartment model. Pharm Res 19:1835–1840
Bonate PL (2011) Pharmacokinetic-pharmacodynamic modeling and simulation. Chapter 8. Nonlinear mixed effects models: practical issues, 2nd edn. Springer, Deerfield, pp 303–358. doi: 10.1007/978-1-4419-9485-1-8
Conflict of interest
None of the authors declare any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honoré, P.H., Joensen, S.J., Olsen, M. et al. Third-space fluid distribution of pemetrexed in non-small cell lung cancer patients. Cancer Chemother Pharmacol 74, 349–357 (2014). https://doi.org/10.1007/s00280-014-2485-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2485-x