Abstract
Background
Perioperative chemotherapy improves the overall survival of resectable gastroesophageal adenocarcinoma (GEA) patients. However, more than 40 % of the patients are not healthy enough to complete their post-operative chemotherapy, and the progression-free survival rate is lower than 35 % at 5 years. In order to optimise neoadjuvant chemotherapy regimen, a pilot study of weekly dose-intensified cisplatin, epirubicin, and paclitaxel (PET) was conducted. The primary objective was a complete resection (R0) rate. Then, a R0 rate ≤80 % was considered as uninteresting, with an expected R0 rate of 92 %. Secondary objectives were the feasibility, safety, histological response rate (Becker score), and survival (Trial registration: NCT01830270).
Methods
Patients with >T1N0M0 GEA were included. Treatment consisted of eight preoperative cycles of weekly PET regimen at 30/50/80 mg/m2 of cisplatin, epirubicin, and paclitaxel, respectively. Primary prophylaxis by granulocyte colony-stimulating factor was administered. Surgery was performed 4–6 weeks following the last cycle of chemotherapy. Using Fleming two-step design with a unilateral alpha type one error of 5 % and a statistical power of 80 %, it would be required to include 68 patients. At planned interim analysis for futility, it was required to observe at least 25 of 29 patients with R0 resection to pursue inclusion. At the second step, it was required to observe at least 61 of 68 patients with R0 resection to conclude for promising activity of the dose-intensified chemotherapy.
Results
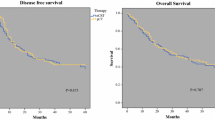
Between May 2011 and January 2013, 29 patients were enrolled. Median age was 62 years (range 39–83 years), and seven (24 %) patients presented signet-ring cell histology. Twenty-seven (93 %) patients underwent surgery. Pathological complete responses (Becker score 1a) were observed in four patients, and nearly complete responses (Becker score 1b) for additional three patients. A R0 rate was achieved for 24 of 29 (82.7 %; 95 % CI 64–94 %) patients. No Becker score 1a/1b response was observed among patients with signet-ring cell GEA. Twenty-one (72 %) patients completed all eight cycles, and 86 % received seven or more cycles. Sixteen (56 %) patients experienced grade 3–4 neutropenia, and five patients had febrile neutropenia. Among non-haematological toxicities, mucositis and fatigue were the most frequent ones. The median-delivered relative dose intensity (DI) was 80 % for cisplatin, 75 % for epirubicin, and 79 % for paclitaxel. However, only 45 % of the patients received at least 80 % of the planned median DI for all three drugs.
Conclusions
Despite high R0 and pathological response rates, neoadjuvant PET chemotherapy did not meet the primary end-point and failed to show an acceptable relative DI. PET chemotherapy is not recommended in resectable GEA patients.



Similar content being viewed by others
References
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Participants MT (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Geneve J, Lasser P, Rougier P (2011) Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29:1715–1721
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997
Biffi R, Fazio N, Luca F, Chiappa A, Andreoni B, Zampino MG, Roth A, Schuller JC, Fiori G, Orsi F, Bonomo G, Crosta C, Huber O (2010) Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. World J Gastroenterol 16:868–874
Moebus V, Jackisch C, Lueck HJ, du Bois A, Thomssen C, Kurbacher C, Kuhn W, Nitz U, Schneeweiss A, Huober J, Harbeck N, von Minckwitz G, Runnebaum IB, Hinke A, Kreienberg R, Konecny GE, Untch M (2010) Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: mature results of an AGO phase III study. J Clin Oncol 28:2874–2880
Untch M, von Minckwitz G, Konecny GE, Conrad U, Fett W, Kurzeder C, Luck HJ, Stickeler E, Urbaczyk H, Liedtke B, Beckmann MW, Salat C, Harbeck N, Muller V, Schmidt M, Hasmuller S, Lenhard M, Nekljudova V, Lebeau A, Loibl S, Fasching PA (2011) PREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel, and CMF versus a standard-dosed epirubicin-cyclophosphamide followed by paclitaxel with or without darbepoetin alfa in primary breast cancer-outcome on prognosis. Ann Oncol 22:1999–2006
Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, Aoki D, Jobo T, Kodama S, Terauchi F, Sugiyama T, Ochiai K (2013) Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 14:1020–1026
Frasci G, D’Aiuto G, Comella P, Thomas R, Botti G, Di Bonito M, De Rosa V, Iodice G, Rubulotta MR, Comella G (2006) Weekly cisplatin, epirubicin, and paclitaxel with granulocyte colony-stimulating factor support vs triweekly epirubicin and paclitaxel in locally advanced breast cancer: final analysis of a sicog phase III study. Br J Cancer 95:1005–1012
Frasci G, D’Aiuto G, Comella P, D’Aiuto M, Di Bonito M, Ruffolo P, Iodice G, Petrillo A, Lastoria S, Oliviero P, Capasso I, Montella M, Siani C, Santangelo M, Vizioli L, Comella G (2010) Preoperative weekly cisplatin, epirubicin, and paclitaxel (PET) improves prognosis in locally advanced breast cancer patients: an update of the Southern Italy Cooperative Oncology Group (SICOG) randomised trial 9908. Ann Oncol 21:707–716
Frasci G, D’Aiuto G, Comella P, Thomas R, Botti G, Di Bonito M, D’Aiuto M, Romano G, Rubulotta MR, Comella G (2005) A 2-month cisplatin–epirubicin–paclitaxel (PET) weekly combination as primary systemic therapy for large operable breast cancer: a phase II study. Ann Oncol 16:1268–1275
Zheng Y, Li F, Qi B, Luo B, Sun H, Liu S, Wu X (2007) Application of perioperative immunonutrition for gastrointestinal surgery: a meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr 16(Suppl 1):253–257
Mariette C, Alves A, Benoist S, Bretagnol F, Mabrut JY, Slim K (2005) Perioperative care in digestive surgery. Guidelines for the French society of digestive surgery (SFCD). Ann Chir 130:108–124
Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P, Jauch KW, Kemen M, Hiesmayr JM, Horbach T, Kuse ER, Vestweber KH (2006) ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clin Nutr 25:224–244
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Bottcher K, Siewert JR, Hofler H (2003) Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 98:1521–1530
Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38:143–151
Moreno Gonzalez E, Gonzalez-Pinto I, Garcia Garcia I, Gomez Sanz R, Loinaz Segurola C, Bercedo Martinez J, Figueroa Andollo J, Palma Carazo F, Marcello Fernandez M (1992) Esophageal resection by cervico-abdominal approach without thoracotomy. Surg Today 22:517–522
Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA, Hofstetter WL, Ilson DH, Keswani RN, Kleinberg LR, Korn WM, Lockhart AC, Meredith K, Mulcahy MF, Orringer MB, Posey JA, Sasson AR, Scott WJ, Strong VE, Varghese TK, Jr, Warren G, Washington MK, Willett C, Wright CD, McMillian NR et al (2013) Gastric cancer, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw 11:531–546
Csendes A, Burdiles P, Rojas J, Braghetto I, Diaz JC, Maluenda F (2002) A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery 131:401–407
Yu W, Choi GS, Chung HY (2006) Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg 93:559–563
Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, Friess H, Hofler H (2011) Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 253:934–939
Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA (1999) Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg 229:303–308
Fields RC, Strong VE, Gonen M, Goodman KA, Rizk NP, Kelsen DP, Ilson DH, Tang LH, Brennan MF, Coit DG, Shah MA (2011) Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer 104:1840–1847
Lorenzen S, Thuss-Patience P, Al-Batran SE, Lordick F, Haller B, Schuster T, Pauligk C, Luley K, Bichev D, Schumacher G, Homann N (2013) Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol 24:2068–2073
Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T, Esaki T, Nagase M, Fujitani K, Yamaguchi K, Ura T, Hamamoto Y, Morita S, Okamoto I, Boku N, Hyodo I (2013) Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 31:4438–4444
Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Awad L, Van Cutsem E (2007) Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol 25:3210–3216
Ferri LE, Ades S, Alcindor T, Chasen M, Marcus V, Hickeson M, Artho G, Thirlwell MP (2012) Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol 23:1512–1517
Fiteni F, Nguyen T, Borg C, Benzidane B, Nerich V, Heyd B, Pivot X, Kim S (2012) Docetaxel, cisplatin and fluorouracil as perioperative chemotherapy in resectable gastroesophageal carcinoma: a retrospective analysis. Ann Oncol 23(Suppl 9):ix224–ix257
Homann N, Pauligk C, Luley K, Werner Kraus T, Bruch HP, Atmaca A, Noack F, Altmannsberger HM, Jager E, Al-Batran SE (2012) Pathological complete remission in patients with oesophagogastric cancer receiving preoperative 5-fluorouracil, oxaliplatin and docetaxel. Int J Cancer 130:1706–1713
Thuss-Patience PC, Hofheinz RD, Arnold D, Florschutz A, Daum S, Kretzschmar A, Mantovani-Loffler L, Bichev D, Breithaupt K, Kneba M, Schumacher G, Glanemann M, Schlattmann P, Reichardt P, Gahn B (2012) Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro-oesophageal adenocarcinoma: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO){dagger}. Ann Oncol 23:2827–2834
Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, Mariette C (2011) The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg 254:684–693; discussion 693
Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C (2009) Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg 250:878–887
Bastholt L, Dalmark M, Gjedde SB, Pfeiffer P, Pedersen D, Sandberg E, Kjaer M, Mouridsen HT, Rose C, Nielsen OS, Jakobsen P, Bentzen SM (1996) Dose–response relationship of epirubicin in the treatment of postmenopausal patients with metastatic breast cancer: a randomized study of epirubicin at four different dose levels performed by the Danish Breast Cancer Cooperative Group. J Clin Oncol 14:1146–1155
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24:2903–2909
Gandara DR, Perez EA, Phillips WA, Lawrence HJ, DeGregorio M (1989) Evaluation of cisplatin dose intensity: current status and future prospects. Anticancer Res 9:1121–1128
Shitara K, Yuki S, Tahahari D, Nakamura M, Kondo C, Tsuda T, Kii T, Tsuji Y, Utsunomiya S, Ichikawa D, Hosokawa A, Ishiguro A, Sakai D, Hironaka S, Oze I, Matsuo K, Muro K (2014) Randomised phase II study comparing dose-escalated weekly paclitaxel vs standard-dose weekly paclitaxel for patients with previously treated advanced gastric cancer. Br J Cancer 110:271–277
http://clinicaltrials.gov/show/NCT01216644. Accessed 5 Jan 2014
http://clinicaltrials.gov/show/NCT01515748. Accessed 5 Jan 2014
Acknowledgments
This work was supported in part by a research grant from the region of Franche Comté. The authors would like to thank the investigators and their team. The authors would like to thank Guadalupe Tizon for English writing assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jary, M., Ghiringhelli, F., Jacquin, M. et al. Phase II multicentre study of efficacy and feasibility of dose-intensified preoperative weekly cisplatin, epirubicin, and paclitaxel (PET) in resectable gastroesophageal cancer. Cancer Chemother Pharmacol 74, 141–150 (2014). https://doi.org/10.1007/s00280-014-2482-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2482-0