Abstract
Background
It remains unclear whether addition of docetaxel to the combination of a platinum and fluoropyrimidine could provide more clinical benefits than doublet chemotherapies in the perioperative treatment for locally advanced gastric/gastro-esophageal junction (LAG/GEJ) cancer in Asia. In this randomized, phase 2 study, we assessed the efficacy and safety of perioperative docetaxel plus oxaliplatin and S-1 (DOS) versus oxaliplatin plus S-1 (SOX) in LAG/GEJ adenocarcinoma patients.
Methods
Patients with cT3–4 Nany M0 G/GEJ adenocarcinoma were randomized (1:1) to receive 4 cycles of preoperative DOS or SOX followed by D2 gastrectomy and another 4 cycles of postoperative chemotherapy. The primary endpoint was major pathological response (MPR).
Results
From Aug, 2015 to Dec, 2019,154 patients were enrolled and 147 patients included in final analysis, with a median age of 60 (26–73) years. DOS resulted in significantly higher MPR (25.4 vs. 11.8%, P = 0.04). R0 resection rate, the 3-year PFS and 3-year OS rates were 78.9 vs. 61.8% (P = 0.02), 52.3 vs. 35% (HR 0.667, 95% CI: 0.432–1.029, Log rank P = 0.07) and 57.5 vs. 49.2% (HR 0.685, 95% CI: 0.429–1.095, Log rank P = 0.11) in the DOS and SOX groups, respectively. Patients who acquired MPR experienced significantly better survival. DOS had similar tolerance to SOX.
Conclusions
Perioperative DOS improved MPR significantly and tended to produce longer PFS compared to SOX in LAG/GEJ cancer in Asia, and might be considered as a preferred option for perioperative chemotherapy and worth further investigation.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the fifth most commonly diagnosed cancer globally and the fourth leading cause of cancer death [1]. In China, it holds the third highest incidence and mortality rates [2]. Moreover, a substantial proportion of Chinese GC patients, approximately 70.8%, are diagnosed with locally advanced disease [3].
The early MAGIC and FNCLCC/FFCD studies established the perioperative chemotherapy modality in local advanced gastric or gastro-esophageal junction (LAG/GEJ) cancer. These studies first proved that pre- and post-operative chemotherapy with epirubicin, cisplatin, and fluorouracil (ECF) and cisplatin and fluorouracil (FP) significantly improved the disease-free survival (DFS) and overall survival (OS) compared with surgery alone [4, 5]. The JCOG0501 study failed to confirm that the perioperative chemotherapy with SP is superior to adjuvant S1 alone [6]. However, two Asian randomized clinical trials demonstrated the advantage of perioperative chemotherapy over postoperative chemotherapy alone. Perioperative oxaliplatin plus S-1 (SOX) and docetaxel combined with oxaliplatin and S-1 (DOS) have been shown to significantly improve the 5-year OS rate compared to adjuvant chemotherapy in RESOLVE and PRODIGY studies, respectively [7, 8]. The perioperative chemotherapy strategy has gained increased evidence and well accepted by most Asian countries.
Although, currently, immune checkpoint inhibitors (ICIs) and targeted therapies are being extensively investigated in the perioperative treatment and have shown promising results in phase II/III clinical trials, chemotherapy remains the cornerstone of neoadjuvant treatment in gastric cancer, and there is still a need to optimize the regimen in terms of efficacy, safety, and convenience. The docetaxel, oxaliplatin, and 5-FU (FLOT) regimen was proved significantly superior to the old ECF in terms of major pathological response (MPR) and survival, and has become the standard perioperative regimen in Europe, as supported by the FLOT4 study [9]. Due to the preference of Asian countries for using oral fluoropyrimidine drugs such as capecitabine or S-1, platinum-based doublet chemotherapies, such as SOX, CapOX, and SP, are the most commonly used regimens in metastatic gastric cancer as well as in the neoadjuvant setting [6, 7, 10, 11], while FLOT has not been widely used in Asia. Among them, the SOX regimen, supported by the RESOLVE study, is considered the preferred perioperative chemotherapy for LAG/GEJ cancer in China [12]. Whether the FLOT regimen and its triplet regimen analogues, consisting of a taxane combined with a platinum and oral fluoropyrimidine drugs, such as DOS, DOX, or DCS, could offer additional clinical benefits compared to their doublet counterparts of SOX, CapOX, or CS, has not been thoroughly investigated yet. In a phase II study with a small sample (N = 74), FLOT did not show a significant advantage in MPR compared to the SOX regimen (20 vs. 34%, P = 0.289), and the survival outcomes of both arms were not reported [13].
As early as 2015, we initiated the MATCH study, a two-component, randomized phase 2 clinical trial (NCT02725424). The aim was to evaluate the efficacy and safety of the DOS and SOX regimens in HER2-negative patients with LAG/GEJ cancer. Additionally, we assessed the therapeutic effects and safety of the combination of the SOX with trastuzumab compared to the SOX alone in HER2-positive patients. Here we present the outcomes of the HER2-negative component of the MATCH study.
Methods
Study design and objective
This was a single-center, open-label, randomized phase II study which aimed to evaluate the efficacy and safety of DOS versus SOX as perioperative chemotherapy in Chinese patients with LAG/GEJ adenocarcinoma conducted at National Cancer Center, China. The protocol was approved by the Ethical Committee of the National Cancer Center/ National Clinical Research Center for Cancer/ Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College (15-077/1004). Clinical trial information: NCT02725424 (https://classic.clinicaltrials.gov/ct2/show/NCT02725424).
Patients
The main inclusion criteria were: (1) age ≥18 years; (2) patients with histologically diagnosed G/GEJ (Siewert II/III type) adenocarcinoma; (3) HER2-negative; (4) cT3–4 Nany M0 by CT (AJCC 7th Edition); (5) no previous treatment including chemotherapy, radiotherapy or gastrectomy; (6) Eastern Cooperative Oncology Group (ECOG) performance status of ≤1; (7) adequate hematological, hepatic and renal functions.
The exclusion criteria were: (1) a diagnosis of other malignant tumors (except for cured skin cancer or cervical carcinoma in situ) within five years; (2) allergic to any ingredients of docetaxel, oxaliplatin, or S-1 medications; (3) distant metastatic disease.
Procedures
Patients were randomly assigned (1:1) to receive perioperative DOS or SOX via an automated interactive web response system. Randomization was stratified by the primary tumor’s location (gastric vs. GEJ cancer), lymph node status (N0 vs. N+), and Lauren classification (intestinal vs. diffuse vs. mixed).
Four cycles of pre- and post-operative chemotherapy with the DOS and SOX regimens were administered. In the DOS group, during each 21-day treatment cycle, patients received intravenous docetaxel 60 mg/m2 and oxaliplatin 100 mg/m2 on day 1, oral S-1 twice a day depending on body surface area (BSA) (BSA < 1.25 m2, 80 mg/day; BSA ≥ 1.25 to <1.5 m2, 100 mg/day and BSA ≥ 1.5 m2, 120 mg/day) from day 1 to day 14. In the SOX group, oxaliplatin was given intravenously at a dose of 130 mg/m2 on day 1, and the S-1 dosing schedule was the same as that of the DOS group, repeated every 21 days. Radical gastrectomy with D2 lymphadenectomy was performed within 4–6 weeks after preoperative chemotherapy.
Preoperative tumor assessment was performed by CT of the chest, abdomen, and pelvis every two cycles according to RECIST version 1.1. Baseline and pre-surgery evaluations such as MRI of the stomach, gastroscopy, and endoscopic ultrasound were recommended but not mandatory. The pathological response was evaluated according to the Becker TRG system by a specified independent pathologist, with tumor regression classified as follows: Grade 1a: complete regression; Grade 1b: <10% residual tumor per tumor bed; Grade 2: 10–50% residual tumor per tumor bed; Grade 3: >50% residual tumor per tumor bed. Adverse events were assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4) both prior to and after the surgery.
Outcomes
The primary endpoint was an MPR analyzed in the modified intention to treat (mITT) population. MPR was defined as pathological complete (TRG1a) and subtotal regression (TRG1b) of the primary tumor according to the Becker TRG system. Secondary endpoints included the 3-year progression free survival (PFS) rate, 3-year OS rate, R0 resection rate, pCR (ypT0N0M0), and safety. PFS was defined as the time from randomization to the first occurrence of disease progression or recurrence based on radiological diagnosis, or death from any cause, whichever occurs first. OS was defined as the time from randomization to death. R0 resection was defined as complete tumor resection without macroscopic or microscopic residual disease.
Statistical analysis
All analyses were performed using SPSS software ver. 25. The MPR in the SOX group was estimated to be 15%, and a calculated sample size of 70 patients per group was needed assuming an improvement in MPR by DOS of 35%, with αlevel of 0.05 (two-sided) and test power of 0.8. Considering a drop-out rate of 5%, 74 patients per group were required. Patients who withdrew their consent before undergoing preoperative chemotherapy were excluded from the analysis. The remaining patients, who were randomized and received any form of study treatment, were included in the mITT population. The MPR, survival, chemotherapy safety, and resection analyses were conducted in the mITT population, while the pathological stage and postoperative complication analyses were performed on patients who underwent surgery (surgery population). PFS and OS were analyzed by the Kaplan–Meier method. Categorical data between these two groups were compared using the chi-squared test. All P-values were 2-sided.
Results
Patient characteristics
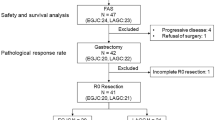
From Aug 2015 to Dec 2019, a total of 154 patients were initially enrolled, with 76 patients randomized to the DOS group and 78 to the SOX group. However, seven patients (DOS 5 patients; SOX 2 patients) withdrew their consent before preoperative treatment. Consequently, the mITT population consisted of 147 patients, 71 patients in the DOS group and 76 patients in the SOX group (Fig. 1). The median age of the patients was 60 (range 26–73) years old, with 78.9% (116/147) males and 21.1% (31/147) females. Baseline characteristics showed no significant difference between the two groups (Table 1). Among these patients, 39 individuals did not accept surgery (DOS 14 patients; SOX 25 patients). The reasons for not proceeding with surgery included disease progression (DOS 1 patient; SOX 4 patients), unresectable conditions due to insufficient tumor shrinkage after chemotherapy (DOS 4 patients; SOX 13 patients), identification of peritoneal metastasis during surgery (DOS 1 patient; SOX 4 patients), refusal of total gastrectomy (DOS 7 patients; SOX 4 patients) and abandonment of treatment (DOS 1 patient).
Pathological findings
In the mITT population, the DOS group demonstrated a significantly higher rate of MPR compared to the SOX group [25.4%, 18/71, (95% CI: 16.7–36.6) vs. 11.8%, 9/76, (95% CI: 6.4–21.0), P = 0.04] (Table 2), and met the primary endpoint of the study. The rates of pCR were 7% (5/71) (95% CI: 3.0–15.4) and 3.9% (3/76) (95% CI: 1.4–11.0) in the respective groups. Moreover, the DOS group had a significantly higher proportion of patients achieving R0 resection [78.9% (56/71) vs. 61.8% (47/76), P = 0.02]. Pathological results are shown in Table 2.
Survival outcomes
Up to Aug 24, 2021, the median follow-up time was 42.4 months (95% CI: 36.340–46.460). The 3-year PFS rates of DOS and SOX groups were 52.3 and 35.0% (HR 0.667, 95% CI: 0.432–1.029, Log-rank P = 0.07, Fig. 2a), respectively, while the 3-year OS rates were 57.5 and 49.2% (HR 0.685, 95% CI: 0.429–1.095, Log-rank P = 0.11, Fig. 2b). Patients who acquired an MPR had significantly higher 3-year PFS rate (89.4 vs. 38.6%, HR 0.076, 95% CI 0.018–0.314, Log-rank P < 0.001) and 3-year OS rate (100 vs. 50.1%, HR 0.024, 95% CI 0.002–0.371, Log-rank P < 0.001) compared to non-MPR patients (Supplementary Fig. 1 and 2). In patients who underwent surgery, distant metastases were more prevalent than local recurrence. However, it was noteworthy that there was no significant difference in the recurrence patterns between the two treatment groups. The DOS group exhibited a lower rate of local recurrence [7.0% (4/57) vs. 13.7% (7/51), P = 0.25] and demonstrated a tendency towards reduced distant metastases [42.1% >(24/57) vs. 58.8% (30/51), P = 0.08] compared with the SOX group (Supplementary Table 1).
Safety profile
In the DOS group and SOX group, 85.9% (61/71) vs. 86.8% (66/76) (P = 0.87) and 76.1% (54/71) vs. 71.1% (54/76) (P = 0.49) of patients respectively completed at least three cycles and four cycles of preoperative chemotherapy, while 56.1% (32/57) and 60.8% (31/51) of the patients respectively completed at least six cycles of perioperative chemotherapy (P = 0.63). The most common grade 3–4 treatment-related adverse events (TRAEs) that occurred in the DOS group and SOX group were neutropenia [8.5% (6/71) vs. 10.5% (8/76), P = 0.67], leucopenia [1.4% (1/71) vs. 5.3% (4/76), P = 0.20], thrombocytopenia [1.4% (1/71) vs. 15.8% (12/76), P = 0.002], anemia [1.4% (1/71) vs. 3.9% (3/76), P = 0.34] and diarrhea [1.4% (1/71) vs. 2.6% (2/76), P = 0.60]. The incidence of grade 1–2 thrombocytopenia was also significantly lower in the DOS group compared to the SOX group [15.5% (11/71) vs. 31.6% (24/76), P = 0.02] (Table 3). During the study, no cases of febrile neutropenia or treatment-related deaths were observed. The DOS regimen didn’t pose a higher risk of surgical complications compared to the SOX regimen (Supplementary Table 2).
Discussion
The patients enrolled in this study were at a relatively more advanced stage, with 30.6% classified as N3 and 37.4% as stage IIIC. These proportions were higher than those reported in previous studies, which were around 16–18 and 15%, respectively [14, 15]. The enrollment criteria for neoadjuvant gastric cancer research vary slightly between Eastern and Western studies. Western studies usually included patients with cT2, while Asian studies predominantly enrolled more advanced patients with T3–4 or those with Borrmann type 4, large type 3, or bulky N2 tumors. In this randomized, phase 2 clinical trial, preoperative DOS significantly improved the MPR rate compared to the SOX regimen (25.4 vs. 11.8%, P = 0.04) in patients with LAG/GEJ cancer. Furthermore, the DOS regimen showed a notable 17.1% increase in the R0 resection rate. The preoperative DOS triplet chemotherapy exhibited higher efficacy in shrinking tumors and showed a promising trend in translating this efficacy into long-term survival benefits. Compared to the SOX regimen, perioperative DOS demonstrated a 33.3% reduction in the risk of progression with numerical improvement in the 3-year PFS rate (Log-rank P = 0.07). Furthermore, although distant metastasis remained the main pattern of recurrence, the DOS regimen exhibited a relative advantage over SOX in reducing local recurrence and controlling distant metastasis. The local recurrence rate was as low as 7%, and the distant metastasis rate was decreased by 16.7%.
Although the DOS regimen demonstrated a higher antitumor activity compared to the SOX regimen, both groups exhibited relatively lower rates of pCR (7 vs. 3.9%) and 3-year PFS (52.3 vs. 35%). In this study, the pCR rate was defined as the proportion of patients with ypT0N0M0 in the mITT population. In the FLOT4 study, which enrolled patients with cT2, pCR rate (defined as ypT0) in mITT population was 16% with FLOT regimen [16]. While in the Asian PRODIGY and RESOLVE studies primarily included patients with cT3–4, the pCR rates (defined as ypT0N0) in surgery population for the DOS and SOX regimens were 10.4 and 5.6%, and the 3-year PFS/DFS rates were 66.3 and 59.4%, respectively [14, 15]. The recent MATTERHORN study also reported an incidence of 7% with ypT0N0 in the FLOT plus placebo arm [17]. The undesirable outcome in this study could be partly due to the enrollment of patients with more locally advanced diseases and a relatively higher proportion of patients who did not undergo surgery. Additionally, as laparoscopic exploration was not mandatory at baseline, some patients with peritoneal metastasis were included.
In addition, a significant improvement in the survival of patients who achieved MPR was observed in this study, which was consistent with previous meta-analysis findings. Gastric cancer patients with residual tumor cells <10% after neoadjuvant chemotherapy experienced better survival outcomes, with a 54% reduction in the risk of death (P < 0.001) [18]. Our results also provided support for the use of MPR as the surrogate primary endpoint in phase 2 studies dealing with neoadjuvant treatment in gastric cancer.
In metastatic GC, modified SOX with a reduced dose of oxaliplatin (100 mg/m2) had a similar efficacy compared to standard dose of CS (cisplatin plus S-1) in the first-line treatment (ORR 55.7 vs. 52.2%) [19]. Based on the finding, we designed the DOS regimen with a reduced dose of oxaliplatin (100 mg/m2) in this study. Actually, the completion rates of 4 cycles of preoperative chemotherapy (76.1 vs. 71.1%) and 6 or more cycles of perioperative chemotherapy (56.1 vs. 60.8%) were similar in both the DOS and SOX groups. Interestingly, the DOS regimen exhibited favorable safety without increasing toxicities compared with SOX. A lower incidence of thrombocytopenia in the DOS group was also observed in this study. The reduced dose of oxaliplatin might partly contribute to the favorable tolerance of the triplet DOS regimen. Immune-mediated reaction is one of the mechanisms of thrombocytopenia induced by oxaliplatin [20]. Steroid was reported to play a role in managing oxaliplatin-induced thrombocytopenia [21]. Therefore, premedication with dexamethasone before docetaxel might help to reduce the occurrence of thrombocytopenia to some extent. The DOS regimen did not increase the risk of postoperative complications or perioperative mortality. When compared with the FLOT4 study, the incidence of some adverse events of DOS in our study was numerically lower, mainly including grade 3–4 neutropenia and diarrhea [9]. This may be associated with the lower dose intensity of docetaxel (20 vs. 25 mg/m2/week) and oxaliplatin (33.3 vs. 42.5 mg/m2/week) [9].
Although FLOT is recommended as one of the perioperative chemotherapy regimens in China, its efficacy and safety have not been fully confirmed in Chinese population. And the inconvenience of intravenous infusion of 5-FU also contributes to the limited widespread use. However, the DOS regimen, with the oral administration of S1, is more convenient to promote. In recent years, several studies have focused on incorporating ICIs into the perioperative treatment for locally advanced GC [17, 22,23,24,25]. In the KEYNOTE-585 phase 3 trial, doublet chemotherapy plus pembrolizumab revealed a significant increase in pCR rate (12.9 vs. 2%, P < 0.0001) compared with chemotherapy plus placebo, while without a significant improvement in event-free survival (EFS) [26]. In the MATTERHORN phase 3 study, combining FLOT with durvalumab significantly increased the pCR rate (19 vs. 7%, P < 0.00001), and survival outcomes have not been reported yet [17]. In view of the favorable safety profile, it was worth further investigating DOS as a partner for ICIs in preoperative treatment.
There are several limitations in the present study. First, it was a single-center study, so the survival advantage needs to be further clarified in a multicenter phase 3 clinical trial. Second, routine diagnostic laparoscopy (DSL) was not mandatory according to the study design. Only CT scans were used to exclude peritoneal metastasis. DSL should be recommended for patients undergoing neoadjuvant treatment. Third, the fact that a relatively high proportion of patients did not receive surgery might bring bias in interpretating the data.
Conclusions
Perioperative DOS improved MPR significantly and tended to produce better PFS compared to SOX in gastric cancer. Triplet DOS also had a favorable safety profile and could be regarded as a preferred option for perioperative chemotherapy in gastric cancer in Asia, and is worth to be further investigated in phase III study.
Data availability
Clinical data will be available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Center. 2022. https://doi.org/10.1016/j.jncc.2022.02.002.
Wang Y, Li Z, Shan F, Miao R, et al. Current status of diagnosis and treatment of early gastric cancer in China-Data from China Gastrointestinal Cancer Surgery Union. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21(2):168–74. https://doi.org/10.3760/cma.j.issn.1671⁃0274.2018.02.010.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. https://doi.org/10.1056/NEJMoa055531.
Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21. https://doi.org/10.1200/JCO.2010.33.059.
Iwasaki Y, Terashima M, Mizusawa J, et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer. 2021;24(2):492–502. https://doi.org/10.1007/s10120-020-01136-7.
Kang Y-K, Kim H-D, Yook JH, et al. Neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 for resectable advanced gastric cancer: Final survival outcomes of the randomized phase 3 PRODIGY trial. 2023 ASCO Annual Meeting; June 2–6; Chicago, IL. J Clin Oncol. 2023;41(16 Suppl):4067.
Zhang XT, Li ZY, Liang H, et al. Overall survival of perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy: An updated analysis of RESOLVE trial. 2023 ESMO Congress; Oct 20–24,2023; Madrid, Spain. Ann Oncol. 2023;34(suppl_2):LBA78.
Al-Batran S-E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. The Lancet. 2019;393(10184):1948–57. https://doi.org/10.1016/S0140-6736(18)32557-1.
Yu Y, Fang Y, Shen Z, et al. Oxaliplatin plus capecitabine in the perioperative treatment of locally advanced gastric adenocarcinoma in combination with D2 gastrectomy: NEO-CLASSIC study. Oncologist. 2019;24(10):1311-e989. https://doi.org/10.1634/theoncologist.2019-0416.
Iwasaki Y, Sasako M, Yamamoto S, et al. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol. 2013;107(7):741–5. https://doi.org/10.1002/jso.23301.
Wang FH, Zhang XT, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41(8):747–95.
Birendra KS, Zhang BY, Zhang H, et al. Neoadjuvant FLOT versus SOX phase II randomized clinical trial for patients with locally advanced gastric cancer. Nat Commun. 2020;11:6093. https://doi.org/10.1038/s41467-020-19965-6.
Zhang XT, Liang H, Li ZY, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22(8):1081–92. https://doi.org/10.1016/S1470-2045(21)00297-7.
Kang YK, Yook JH, Park YK, et al. PRODIGY: A phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. J Clin Oncol. 2021;39(26):2903–13. https://doi.org/10.1200/JCO.20.02914.
Al-Batran S-E, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17(12):1697–708. https://doi.org/10.1016/S1470-2045(16)30531-9.
Janjigian YY, Al-Batran S-E, Wainberg ZA, et al. Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): interim results of the global, phase III MATTERHORN study. 2023 ESMO Congress; Oct 20–24,2023; Madrid, Spain. Ann Oncol. 2023;34(suppl_2):LBA73.
Tomasello G, Petrelli F, Ghidini M, et al. Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: a meta-analysis of 17 published studies. Eur J Surg Oncol. 2017;43(9):1607–16. https://doi.org/10.1016/j.ejso.2017.03.001.
Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26(1):141–8. https://doi.org/10.1093/annonc/mdu472.
Taleghani BM, Meyer O, Fontana S, et al. Oxaliplatin-induced immune pancytopenia. Transfusion. 2005;45(5):704–8. https://doi.org/10.1111/j.1537-2995.2005.04373.x.
DL Jardim, CA Rodrigues, YAS. Novis, et al.Oxaliplatin-related thrombocytopenia. Ann Oncol. 2012;23:1937–42. https://doi.org/10.1093/annonc/mds074.
Jiang H, Yu X, Li N, et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J Immunother Cancer. 2022;10(3): e003635. https://doi.org/10.1136/jitc-2021-003635.
Sun W, Veeramachaneni N, AI-Rajabi RMdT, et al. A phase II study of perioperative pembrolizumab plus mFOLFOX in patients with potentially resectable esophagus, gastroesophageal junction (GEJ) and stomach adenocarcinoma. Cancer Med. 2023;12(5):16098–107. https://doi.org/10.1002/cam4.6263.
Liu Z, Liu N, Zhou Y, et al. Efficacy and safety of camrelizumab combined with FLOT versus FLOT alone as neoadjuvant therapy in patients with resectable locally advanced gastric and gastroesophageal junction adenocarcinoma who received D2 radical gastrectomy: Data update. 2022 ASCO Annual Meeting; June 3–7; Chicago, IL. J Clin Oncol. 2022;40(16 Suppl):e16044.
Al-Batran S-E, Lorenzen S, Thuss-Patience PC, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: Interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. 2022 ASCO Annual Meeting; June 3–7; Chicago, IL, USA. J Clin Oncol. 2022;40(16 Suppl):4003.
Shitara K, Rha SY, Wyrwicz LS, et al. Pembrolizumab plus chemotherapy vs chemotherapy as neoadjuvant and adjuvant therapy in locally-advanced gastric and gastroesophageal junction cancer: The phase III KEYNOTE-585 study. 2023 ESMO Congress; Oct 20–24, 2023; Madrid, Spain. Ann Oncol. 2023;34(suppl_2):LBA74.
Acknowledgements
Aiping Zhou had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
There is no funding for this study.
Author information
Authors and Affiliations
Contributions
ZAP, ZDB, GSG and TYT designed, carried out and supervised the study, and approved the final version of the manuscript. ZAP, ZDB, GSG, TYT, XYB, ZW, DCX, ZYX, SK, SYK, XQ and JZC participated in patient recruitment and informed consent harvest. JLM was responsible for imaging evaluation. DLZ was responsible for the evaluation of gastroscopy and endoscopic ultrasound. ZYL did pathological evaluation. ZAP, ZW, DCX, SYK and JZC collected data. ZAP and JZC analyzed and interpreted data. JZC drafted the manuscript, and edited the paper for English grammar and writing. ZAP did Critical revision of the manuscript for important intellectual content. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The protocol was approved by the Ethical Committee of the National Cancer Center/ National Clinical Research Center for Cancer/ Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College (15–077/1004). Clinical trial information: NCT02725424. Written informed consent was obtained from all individual participants included in the study. This study followed the CONSORT reporting guideline for randomized studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, Z., Xie, Y., Zhang, W. et al. Perioperative chemotherapy with docetaxel plus oxaliplatin and S-1 (DOS) versus oxaliplatin plus S-1 (SOX) for the treatment of locally advanced gastric or gastro-esophageal junction adenocarcinoma (MATCH): an open-label, randomized, phase 2 clinical trial. Gastric Cancer 27, 571–579 (2024). https://doi.org/10.1007/s10120-024-01471-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-024-01471-z