Abstract
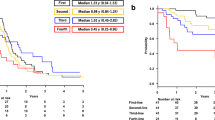
Based on centroblast frequency, follicular lymphoma (FL) is subdivided into grades 1-2, 3A, and 3B. Grade FL3A frequently coexists with FL1-2 (FL1-2-3A). Based on clinical trials, FL1-2 is treated with rituximab (R) or obinutuzumab plus bendamustine (B) or CHOP, while FL3B is treated with R-CHOP. In contrast, there are little data guiding therapy in FL3A. We present a retrospective, multicenter analysis of 95 FL3A or FL1-2-3A and 203 FL1-2 patients treated with R-CHOP or R-B first-line. R-CHOP facilitated a higher response rate (95% versus 76%) and longer overall survival (OS) (3-year OS 89% versus 73%, P = 0.008) in FL3A or FL1-2-3A, whereas the difference in progression-free survival (PFS) did not reach statistical significance. While transformation rates into aggressive lymphoma were similar between both groups, there were more additional malignancies after R-B compared with R-CHOP (6 versus 2 cases). In FL1-2, R-B achieved a higher 3-year PFS (79% versus 47%, P < 0.01), while there was no significant difference regarding OS or transformation. With the limitations of a retrospective analysis, these results suggest a benefit for R-CHOP over R-B in FL3A or FL1-2-3A. Confirmatory data from prospective clinical trials are needed.


Similar content being viewed by others
Availability of data and material
The datasets generated and analyzed during the current study are not publicly available due to personal data protection reasons but are available from the corresponding author on reasonable request.
References
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman J (2017) WHO classification of tumours of haematopoietic and lymphoid tissues. In: WHO classification of tumours, Lyon. WHO reference number: 17024002. 266–279
Ott G, Katzenberger T, Lohr A, Kindelberger S, Rüdiger T, Wilhelm M, Kalla J, Rosenwald A, Müller JG, Michaela Ott M, Müller-Hermelink HK (2002) Cytomorphologic, immunohistochemical, and cytogenetic profiles of follicular lymphoma: 2 types of follicular lymphoma grade 3. Blood. 99(10):3806–3812
Horn H, Schmelter C, Leich E, Salaverria I, Katzenberger T, Ott MM, Kalla J, Romero M, Siebert R, Rosenwald A, Ott G (2011) Follicular lymphoma grade 3B is a distinct neoplasm according to cytogenetic and immunohistochemical profiles. Haematologica. 96:1327–1334
Horn H, Kohler C, Witzig R, Kreuz M, Leich E, Klapper W, Hummel M, Loeffler M, Trümper L, Spang R, Rosenwald A, Ott G (2018) Gene expression profiling reveals a close relationship between follicular lymphoma grade 3A and 3B, but distinct profiles of follicular lymphoma grade 1 and 2. Haematologica. 103(7):1182–1190
Koch K, Hoster E, Ziepert M, Unterhalt M, Ott G, Rosenwald A, Hansmann ML, Bernd W, Stein H, Pöschel V, Dreyling M, Trümper L, Löffler M, Schmitz N, Hiddemann W, Pfreundschuh M, Klapper W (2016) Clinical, pathological and genetic features of follicular lymphoma grade 3A: a joint analysis of the German low-grade and high-grade lymphoma study groups GLSG and DSHNHL. Ann Oncol 27(7):1323–1329
Pastore A, Jurinovic V, Kridel R, Hoster E, Staiger AM, Szczepanowski M, Pott C, Kopp N, Murakami M, Horn H, Leich E, Moccia AA, Mottok A, Sunkavalli A, Van Hummelen P, Ducar M, Ennishi D, Shulha HP, Hother C, Connors JM, Sehn LH, Dreyling M, Neuberg D, Möller P, Feller AC, Hansmann ML, Stein H, Rosenwald A, Ott G, Klapper W, Unterhalt M, Hiddemann W, Gascoyne RD, Weinstock DM, Weigert O (2015) Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol 16(9):1111–1122
Casulo C (2018) Risk stratification in follicular lymphoma. Best Pract Res Clin Haematol 31(1):15–22
Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF, Townsend W, Trněný M, Wenger M, Fingerle-Rowson G, Rufibach K, Moore T, Herold M, Hiddemann W (2017) Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med 377(14):1331–1344
Salles G, Mounier N, De Guibert S, Morschhauser F, Doyen C, Rossi JF, Haioun C, Brice P, Mahé B, Bouabdallah R, Audhuy B, Ferme C, Dartigeas C, Feugier P, Sebban C, Xerri L, Foussard C (2008) Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood. 112:4824–4831
Hiddemann W, Barbui AM, Canales MA, Cannell PK, Collins GP, Dürig J, Forstpointner R, Herold M, Hertzberg M, Klanova M, Radford J, Seymour JF, Tobinai K, Trotman J, Burciu A, Fingerle-Rowson G, Wolbers M, Nielsen T, Marcus RE (2018) Immunochemotherapy with obinutuzumab or rituximab for previously untreated follicular lymphoma in the GALLIUM study: influence of chemotherapy on efficacy and safety. J Clin Oncol 36(23):2395–2404
Flinn IW, Van Der Jagt R, Kahl BS, Wood P, Hawkins TE, MacDonald D, Hertzberg M, Kwan YL, Simpson D, Craig M, Kolibaba K, Issa S, Clementi R, Hallman DM, Munteanu M, Chen L, Burke JM (2014) Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 123(19):2944–2952
Rummel MJ, Niederle N, Maschmeyer G, Banat GA, Von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E, Dürk H, Ballo H, Stauch M, Roller F, Barth J, Hoelzer D, Hinke A, Brugger W (2013) Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381(9873):1203–1210. https://doi.org/10.1016/S0140-6736(12)61763-2 [Internet]
Roisman A, Castellano G, Navarro A, Gonzalez-Farre B, Pérez-Galan P, Esteve-Codina A, Dabad M, Heath S, Gut M, Bosio M, Bellot P, Salembier P, Oliveras A, Slavutsky I, Magnano L, Horn H, Rosenwald A, Ott G, Aymerich M, López-Guillermo A, Jares P, Martín-Subero JI, Campo E, Hernández L (2019) Differential expression of long non-coding RNAs are related to proliferation and histological diversity in follicular lymphomas. Br J Haematol 184(3):373–383
Fisher RL, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP (2005) New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol 23(33):8447–52
Ladetto M, De Marco F, Benedetti F, Vitolo U, Parti C, Rambaldi A, Pulsoni A, Musso M, Liberati AM, Olivieri A, Gallamini A, Pogliani E, Scalabrini DR, Callea V, Di Raimondo F, Pavone V, Tucci A, Cortelazzo S, Levis A, Boccadoro M, Majolino I, Pileri A, Gianni AM, Passera R, Corradini P, Tarella C (2008) Prospective, multicenter randomized GITMO/IIL trial comparing intensive (R-HDS) versus conventional (CHOP-R) chemoimmunotherapy in high-risk follicular lymphoma at diagnosis: the superior disease control of R-HDS does not translate into an overall survival advantage. Blood 111(8):4004–13
Mondello P, Steiner N, Willenbacher W, Cerchione C, Nappi D, Mauro E, Ferrero S, Cuzzocrea S, Mian M (2018) Bendamustine plus rituximab versus R-CHOP as first-line treatment for patients with follicular lymphoma grade 3A: evidence from a multicenter, retrospective study. Oncologist 23(4):454–460
Shah NN, Szabo A, Saba R, Strelec L, Kodali D, Vaughn JL, Esan O, Yang DT, Mato AR, Kanate AS, Olteanu H, Hamadani M, Fenske TS, Kenkre VP, Svoboda J, Cashen AF, Epperla N (2019) Multicenter analysis of advanced stage grade 3A follicular lymphoma outcomes by frontline treatment regimen. Clin Lymphoma Myeloma Leuk 19(2):95–102
Steen CB, Leich E, Myklebust JH, Lockmer S, Wise JF, Wahlin BE, Østenstad B, Liestøl K, Kimby E, Rosenwald A, Smeland EB, Holte H, Lingjærde OC, Brodtkorb M (2019) A clinico-molecular predictor identifies follicular lymphoma patients at risk of early transformation after first-line immunotherapy. Haematologica. 104(10):E460–E464
Montoto S, Davies AJ, Matthews J, Calaminici M, Norton AJ, Amess J, Vinnicombe S, Waters R, Rohatiner AZS, Lister TA (2007) Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol 25(17):2426–2433
Sarkozy C, Trneny M, Xerri L, Wickham N, Feugier P, Leppa S, Brice P, Soubeyran P, Da Silva MG, Mounier C, Offner F, Dupuis J, Caballero D, Canioni D, Paula M, Delarue R, Zachee P, Seymour J, Salles G, Tilly H (2016) Risk factors and outcomes for patients with follicular lymphoma who had histologic transformation after response to first-line immunochemotherapy in the PRIMA trial. J Clin Oncol 34(22):2575–82
Rummel MJ, Maschmeyer G, Ganser A, Heider A, von Gruenhagen U, Losem C, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E, Dürk HA, Ballo H, Stauch M, Blau W, Burchardt A, Barth J, Kauff F, Brugger W (2017) Bendamustine plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent lymphomas: nine-year updated results from the StiL NHL1 study. J Clin Oncol 35:7501
Flinn IW, Van Der Jagt R, Kahl B, Wood P, Hawkins T, MacDonald D, Simpson D, Kolibaba K, Issa S, Chang J, Trotman J, Hallman D, Chen L, Burke JM (2019) First-line treatment of patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: results of the BRIGHT 5-year follow-up study. J Clin Oncol 37(12):984–991. https://doi.org/10.1200/JCO.18.00605 [internet]
Jurinovic V, Passerini V, Oestergaard MZ, Knapp A, Mundt K, Araf S, Richter J, Fitzgibbon J, Klapper W, Marcus RE, Davies A, Herold M, Hiddemann W, Unterhalt M, Hoster E, Weigert O (2019) Evaluation of the m7-FLIPI in patients with follicular lymphoma treated within the Gallium trial: EZH2 mutation status may be a predictive marker for differential efficacy of chemotherapy. Blood. 134:122
Author information
Authors and Affiliations
Consortia
Contributions
MP collected and analyzed the data and wrote the first draft of the manuscript. AM, AV, PLR, GM, CH, TW, MWH, SB, MH, DK, CK, VV, AM, JM, HS, RM, GPK, FK, EH, and UK collected the data. AS guided the statistical analysis. KK and WK performed the reference pathology. All authors commented on previous versions of the manuscript and revised the paper critically. CWS designed the research, overlooked the study, commented on previous versions of the manuscript, and revised the paper critically.
Corresponding author
Ethics declarations
Conflict of interest
AV has been on Advisory Boards for Amgen, Roche, Kite/Gilead, and Novartis and received travel support and speaker honoraria from Amgen, Roche, Kite/Gilead, and Janssen. PLR has been on the Advisory Board for Roche. GM has received honoraria for lectures from Gilead, Janssen Cilag, AMGEN, Merck-Serono, Bristol-Myers Squibb, and AstraZeneca and travel grants from Janssen Cilag. TW has received travel grants and has been on the Advisory Board for Roche. SB reports consulting fees from Roche and AbbVie, research funding from Roche, Janssen, AbbVie, and Celgene, and honoraria from Roche, AbbVie, Janssen, Novartis, and Becton Dickinson. HS has received speaker honoraria and has been on the Advisory Boards for Lilly, MSD, Roche, Novartis, BMS, Basilea, Merck Serono, and Servier and has received research support from Sanofi. RM has received speaker honoraria and has been on the Advisory Boards for Janssen, Merck, Eusa, Novartis, and Kite/Gilead and has received travel grants from Kite/Gilead. UK has received travel support, speaker support and speaker honoraria from Roche and travel support and speaker honoraria from Mundipharma. WK has received research grants from Roche, Takeda, Amgen and Regeneron. SWS has received speaker honoraria from Gilead, Janssen and Pfizer and has been on the Advisory Boards for Roche, Novartis, Janssen, Hexal, Gilead, Takeda, Daiichi Sankyo, Celgene, Bristol-Myers Squibb, and Merck Serono. All remaining authors have declared no conflicts of interest.
Ethical approval
The retrospective analysis was approved by the ethics committee of the Berlin Chamber of Physicians. The study was conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki.
Consent to participate
As this is a retrospective analysis of patient data, no additional informed consent was required. Patient data was collected and analyzed in anonymized form. Dates of birth were provided as five-year intervals in order to avoid patient identification (i.e., 1950–1954 for a patient born in 1952).
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1a
Kaplan-Meier plot of time-to-next treatment for patients with grade 3A or 1-2-3A FL with R-CHOP versus R-Bendamustine (DOCX 35 kb).
Supplementary Fig. 1b
Kaplan-Meier plot of time-to-next treatment for patients with grade 1-2 FL with R-CHOP versus R-Bendamustine (DOCX 39 kb).
Supplementary Table 1
(DOCX 15 kb).
Supplementary Table 2
(DOCX 15 kb).
Supplementary Table 3
(DOCX 15 kb).
Rights and permissions
About this article
Cite this article
Pouyiourou, M., Meyer, A., Stroux, A. et al. First-line treatment with R-CHOP or rituximab-bendamustine in patients with follicular lymphoma grade 3A—results of a retrospective analysis. Ann Hematol 99, 2821–2829 (2020). https://doi.org/10.1007/s00277-020-04171-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04171-7