Abstract
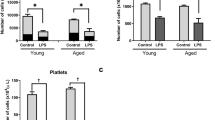
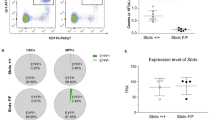
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening systemic hyperinflammatory disorder. We found recently that repeated lipopolysaccharide (LPS) treatment induces HLH-like features in senescence-accelerated mice (SAMP1/TA-1) but not in senescence-resistant control mice (SAMR1). In this study, we analyzed the dynamics of hematopoiesis in this mouse model of HLH. When treated repeatedly with LPS, the numbers of myeloid progenitor cells (CFU-GM) and B-lymphoid progenitor cells (CFU-preB) in the bone marrow (BM) rapidly decreased after each treatment in both strains. The number of CFU-GM in SAMP1/TA-1 and SAMR1, and of CFU-preB in SAMR1, returned to pretreatment levels by 7 days after each treatment. However, the recovery in the number of CFU-preB in SAMP1/TA-1 was limited. In both strains, the BM expression of genes encoding positive regulators of myelopoiesis (granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), and interleukin (IL)-6), and negative regulators of B lymphopoiesis (tumor necrosis factor (TNF)-α) was increased. The expression of genes encoding positive regulators of B lymphopoiesis (stromal-cell derived factor (SDF)-1, IL-7, and stem cell factor (SCF)) was persistently decreased in SAMP1/TA-1 but not in SAMR1. Expression of the gene encoding p16INK4a and the proportion of β-galactosidase-positive cells were increased in cultured stromal cells obtained from LPS-treated SAMP1/TA-1 but not in those from LPS-treated SAMR1. LPS treatment induced qualitative changes in stromal cells, which comprise the microenvironment supporting appropriate hematopoiesis, in SAMP1/TA-1; these stromal cell changes are inferred to disrupt the dynamics of hematopoiesis. Thus, hematopoietic tissue is one of the organs that suffer life-threatening damage in HLH.





Similar content being viewed by others
References
Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G, for the Histiocyte Society (2007) HLH-2004: Diagnostic and therapeutic guideline for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 48:124–131
Créput C, Galicier L, Buyse S, Azoulay E (2008) Understanding organ dysfunction in hemophagocytic lymphohistiocytosis. Intensive Care Med 34:1177–1187
Janka GE (2012) Familial and acquired hemphagohistiocytosis. Annu Rev Med 63:233–246
Stepp SE, Dufourcq-Lagelouse R, Le Deist F et al (1999) Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 286:1957–1959
Voskoboinik I, Smyth MJ, Trapani JA (2006) Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol 6:940–952
Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachée-Chardin M, Chedeville G, Tamary H, Minard-Colin V, Vilmer E, Blanche S, le Deist F, Fischer A, de Saint Basile G (2003) Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. 115:461–473
zur Stadt U, Schmidt S, Kasper B et al (2005) Linkage of familiar hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutation in syntaxin 11. Hum Mol Genet 14:827–834
zur Stadt U, Rohr J, Seifert W et al (2009) Familiar hemophagocytic lymphohistiocytosis type 5(FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet 85:482–492
Usmani GN, Woda BA, Newburger PE (2013) Advances in understanding the pathogenesis of HLH. Br J Haematol 161:609–622
Tsuboi I, Harada T, Hirabayashi Y, Aizawa S (2019) Senescence-accelerated mice (SAMP1/TA-1) treated repeatedly with lipopolysaccharide develop a condition that resembles hemophagocytic lymphohistiocytosis. Haematologica. 104:1995–2005
Canna SW, Wrobel J, Chu N, Kreiger PA, Paessler M, Behrens EM (2013) Interferon-γ mediates anemia but is dispensable for fulminant toll-like receptor 9-induced macrophage activation syndrome and hemophagocytosis in mice. Arthritis Rheum 65:1764–1775
Janka GE (2007) Familial and acquired hemophagocytic lymphohistiocytosis. Eur J Pediatr 166:95–109
Machowicz R, Janka G, Wiktor-Jedrzejczak W (2017) Similar but not the same: differential diagnosis of HLH and sepsis. Crit Rev Oncol Hematol 114:1–12
Schuettpelz LG, Link DC (2013) Regulation of hematopoietic stem cell activity by inflammation. Front Immunol 4:204
Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamuro T (1981) A new murine model of accelerated senescence. Mech Ageing Dev 17:183–194
Tsuboi I, Morimoto K, Horie T, Mori KJ (1991) Age-related changes in various hematopoietic progenitor cells in senescence-accelerated (SAM-P) mice. Exp Hematol 19:874–877
Tsuboi I, Harada T, Hirabayashi Y, Kanno J, Aizawa S (2016) Differential regulation of lympho-myelopoiesis by stromal cells in the early and late phases in BALB/c mice repeatedly exposed to lipopolysaccharide. Biol Pharm Bull 39:1939–1947
Tsuboi I, Harada T, Hirabayashi Y, Kanno J, Inoue T, Aizawa S (2012) Age-related decline of mast cell regeneration in senescence-accelerated mice (SAMP1) after chemical myeloablation due to senescent stromal cell impairment. Exp Biol Med 237:1289–1297
Tsuboi I, Hirabayashi Y, Harada T, Hiramoto M, Kanno J, Inoue T, Aizawa S (2008) Predominant regeneration of B-cell lineage, instead of myeloid linage, of the bone marrow after 1 Gy whole-body irradiation in mice: role of differential cytokine expression between B-cell stimulation by IL10, Flt3 ligand and IL7 and myeloid suppression by GM-CSF and SCF. Radiat Res 170:15–22
Tsuboi I, Hirabayashi Y, Harada T, Koshinaga M, Kawamata T, Kanno J, Inoue T, Aizawa S (2008) Role of hematopoietic microenvironment in prolonged impairment of B cell regeneration in age-related stromal-cell-impaired SAMP1 mouse: effects of a single dose of 5-fluorouracil. J Appl Toxicol 28:797–805
Dexter TM, Allen TD, Lajtha LG (1977) Condition controlling the proliferation of hematopoietic stem cells in vitro. J Cell Physiol 91:335–344
Mori KJ, Fujitake H, Okubo H, Dexter TM, Ito Y (1979) Quantitative development of adherent cell colonies in bone marrow cell culture in vitro. Exp Hematol 7:171–176
Aizawa S, Yaguchi M, Nakano M et al (1994) Hematopoietic supportive function of human bone marrow stromal cell lines established by a recombinant SV40-adenovirus vector. Exp Hematol 22:482–487
Takizawa H, Boettcher S, Manz MG (2012) Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 119:2991–3002
Ueda Y, Kondo M, Kelsoe G (2005) Inflammation and reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med 201:1771–1780
Pascutti MF, Erkelen MN, Nolte MA (2016) Impact of viral infections on hematopoiesis: From beneficial to detrimental effects on bone marrow output. Front Immunol 7:364
Schepers K, Campbell TB, Passegué E (2015) Normal and leukemic niches: Insight and therapeutic opportunities. Cell Stem Cell 16:254–267
Nombela-Arrieta C, Isringhausen S (2017) The role of the bone marrow stromal compartment in the hematopoietic response to microbial infections. Front Immunol 7:689
Cain D, Kondo M, Chen H, Kelsoe G (2009) Effect of acute and chronic inflammation on B-cell development and differentiation. J Invest Dermatol 129:266–277
Carbonneau CL, Desparas G, Rojas-Sutterlin S, Fortin A, Le O, Hoang T, Beauséjour CM (2012) Ionizing radiation-induced expression of INK4a/ARF in murine bone marrow –derived stromal cell populations interferes with bone marrow homeostasis. Blood. 119:717–726
Feng X, Feng G, Xing J, Shen B, Tan W, Huang D, Lu X, Tao T, Zhang J, Li L, Gu Z (2014) Repeated lipopolysaccharide stimulation promotes cellular senescence in human dental pulp stem cells (DPSCs). Cell Tissue Res 356:369–380
Kovtonyuk LV, Fritsch K, Feng X, Manz MG, Takizawa H (2016) Inflamm-aging of hematopoiesis, hematopoietic stem cells, and the bone marrow microenvironment. Front Immunol 7:502
Acknowledgments
We thank Sonoko Araki and Miyuki Yuda for technical assistance.
Funding
This work has been supported in part by a Grant-in Aid for Science Research C from the Japan Society for the Promotion of Science (Grant Number JP18K06846).
Author information
Authors and Affiliations
Contributions
All authors participated in the design and interpretation of the study, analysis of the data, and review of the manuscript; IT, TH, and SA conducted the experiments; IT, TI, YH, and SA wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsuboi, I., Harada, T., Hirabayashi, Y. et al. Dynamics of hematopoiesis is disrupted by impaired hematopoietic microenvironment in a mouse model of hemophagocytic lymphohistiocytosis. Ann Hematol 99, 1515–1523 (2020). https://doi.org/10.1007/s00277-020-04095-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04095-2