Abstract
Purpose
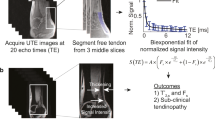
Although enthesitis is a hallmark of several rheumatologic conditions, current imaging methods are still unable to characterize entheses changes because of the corresponding short transverse relaxation times (T2). A growing number of MR studies have used Ultra-High Field (UHF) MRI in order to assess low-T2 tissues e.g., tendon but never in humans. The purpose of the present study was to assess in vivo the enthesis of the quadriceps tendon in healthy subjects using UHF MRI.
Methods
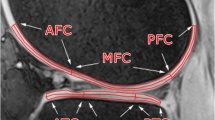
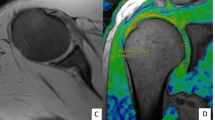
Eleven healthy subjects volunteered in an osteoarthritis imaging study. The inclusion criteria were: no knee trauma, Lequesne index = 0, less than 3 h of sport activities per week, and Kellgren and Lawrence grade = 0. 3D MR images were acquired at 7 T using GRE sequences and a T2* mapping. Regions of interest i.e., trabecular bone, subchondral bone, enthesis, and tendon body were identified, and T2* values were quantified and compared.
Results
Quadriceps tendon enthesis was visible as a hyper-intense signal. The largest and the lowest T2* values were quantified in the subchondral bone region and the tendon body respectively. T2* value within subchondral bone was significantly higher than T2* value within the enthesis. T2* in subchondral bone region was significantly higher than the whole tendon body T2*.
Conclusion
A T2* gradient was observed along the axis from the enthesis toward the tendon body. It illustrates different water biophysical properties. These results provide normative values which could be used in the field of inflammatory rheumatologic diseases and mechanical disorders affecting the tendon.


Similar content being viewed by others
Data availability
All the dataset are available locally at CRMBM-CEMEREM, Timone Hospital, Marseille France.
References
Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR (2002) The skeletal attachment of tendons—tendon ‘entheses.’ Comp Biochem Physiol A: Mol Integr Physiol 133:931–945. https://doi.org/10.1016/s1095-6433(02)00138-1
Rossetti L, Kuntz LA, Kunold E et al (2017) The microstructure and micromechanics of the tendon–bone insertion. Nature Mater 16:664–670. https://doi.org/10.1038/nmat4863
Milz S, Benjamin M, Putz R (2005) Molecular parameters indicating adaptation to mechanical stress in fibrous connective tissue. Adv Anat Embryol Cell Biol 178:1–71
Salah MM, Yong YR, Poh WT, Chong LR (2019) Multiple spontaneous tendon ruptures from enthesis failure in primary hyperparathyroidism: a case report and review of imaging findings. Skeletal Radiol 48:1279–1287. https://doi.org/10.1007/s00256-018-3092-4
Wu W, Wang C, Ruan J, Wang H, Huang Y, Zheng W, Chen F (2019) Simultaneous spontaneous bilateral quadriceps tendon rupture with secondary hyperparathyroidism in a patient receiving hemodialysis: a case report. Medicine 98:e14809. https://doi.org/10.1097/MD.0000000000014809
Resnick D, Niwayama G (1976) Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology 119:559–568. https://doi.org/10.1148/119.3.559
Vaishya R, Vijay V, Nwagbara IC, Agarwal AK (2017) Diffuse idiopathic skeletal hyperostosis (DISH)–a common but less known cause of back pain. J Clin Orthop Trauma 8:191–196. https://doi.org/10.1016/j.jcot.2016.11.006
Herrou J, Picaud AS, Lassalle L et al (2022) Prevalence of enthesopathies in adults with X-linked hypophosphatemia: analysis of risk factors. J Clin Endocrinol Metab 107:e224–e235. https://doi.org/10.1210/clinem/dgab580
Braun J, Bollow M, Eggens U, König H, Distler A, Sieper J (1994) Use of dynamic magnetic resonance imaging with fast imaging in the detection of early and advanced sacroiliitis in spondylarthropathy patients. Arthritis Rheum 37:1039–1045. https://doi.org/10.1002/art.1780370709
Rudwaleit M, Jurik AG, Hermann K-GA et al (2009) Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 68:1520–1527. https://doi.org/10.1136/ard.2009.110767
McGonagle D, Conaghan PG, O’Connor P et al (1999) The relationship between synovitis and bone changes in early untreated rheumatoid arthritis: a controlled magnetic resonance imaging study. Arthritis Rheum 42:1706–1711. https://doi.org/10.1002/1529-0131(199908)42:8%3c1706::AID-ANR20%3e3.0.CO;2-Z
Chen B, Zhao Y, Cheng X et al (2018) Three-dimensional ultrashort echo time cones (3D UTE-Cones) magnetic resonance imaging of entheses and tendons. Magn Reson Imaging 49:4–9. https://doi.org/10.1016/j.mri.2017.12.034
Juras V, Zbyn S, Pressl C, Valkovic L, Szomolanyi P, Frollo I, Trattnig S (2012) Regional variations of T 2 * in healthy and pathologic achilles tendon in vivo at 7 tesla: preliminary results: T 2 * in healthy and pathologic AT at 7 T. Magn Reson Med 68:1607–1613. https://doi.org/10.1002/mrm.24136
Trudel G, Melkus G, Cron GO et al (2017) Imaging of the rabbit supraspinatus enthesis at 7 Tesla: a 4-week time course after repair surgery and effect of channeling: enthesis reformation at 7T: experimental study. J Magn Reson Imaging 46:461–467. https://doi.org/10.1002/jmri.25589
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM (2012) FSL. Neuroimage 62(2):782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
Ogier A, Sdika M, Foure A, Le Troter A, Bendahan D (2017) Individual muscle segmentation in MR images: A 3D propagation through 2D non-linear registration approaches. Conf Proc IEEE Eng Med Biol Soc 2017:317–320. https://doi.org/10.1109/EMBC.2017.8036826
Nöbauer-Huhmann I-M, Pretterklieber M, Erhart J et al (2012) Anatomy and variants of the triangular fibrocartilage complex and its MR appearance at 3 and 7T. Semin Musculoskelet Radiol 16:93–103. https://doi.org/10.1055/s-0032-1311761
Toumi H, Larguech G, Filaire E, Pinti A, Lespessailles E (2012) Regional variations in human patellar trabecular architecture and the structure of the quadriceps enthesis: a cadaveric study: Patellar trabecular architecture and the structure of the quadriceps enthesis. J Anat 220:632–637. https://doi.org/10.1111/j.1469-7580.2012.01500.x
Juras V, Mlynarik V, Szomolanyi P, Valkovič L, Trattnig S (2019) Magnetic resonance imaging of the musculoskeletal system at 7T: morphological Imaging and Beyond. Top Magn Reson Imaging 28:125–135. https://doi.org/10.1097/RMR.0000000000000205
Chang G, Boone S, Martel D et al (2017) MRI assessment of bone structure and microarchitecture. J Magn Reson Imaging 46:323–337. https://doi.org/10.1002/jmri.25647
Benjamin M, Milz S, Bydder GM (2008) Magnetic resonance imaging of entheses. Part 1. Clin Radiol 63:691–703. https://doi.org/10.1016/j.crad.2007.12.011
Benjamin M, Toumi H, Suzuki D, Redman S, Emery P, McGonagle D (2007) Microdamage and altered vascularity at the enthesis–bone interface provides an anatomic explanation for bone involvement in the HLA–B27–associated spondylarthritides and allied disorders. Arthritis Rheum 56:224–233. https://doi.org/10.1002/art.22290
Chen B, Cheng X, Dorthe EW et al (2019) Evaluation of normal cadaveric Achilles tendon and enthesis with ultrashort echo time (UTE) magnetic resonance imaging and indentation testing. NMR Biomed 32:e4034. https://doi.org/10.1002/nbm.4034
Juras V, Apprich S, Szomolanyi P, Bieri O, Deligianni X, Trattnig S (2013) Bi-exponential T2 analysis of healthy and diseased Achilles tendons: an in vivo preliminary magnetic resonance study and correlation with clinical score. Eur Radiol 23:2814–2822. https://doi.org/10.1007/s00330-013-2897-8
Diaz E, Chung CB, Bae WC et al (2012) Ultrashort echo time spectroscopic imaging (UTESI): an efficient method for quantifying bound and free water. NMR Biomed 25:161–168. https://doi.org/10.1002/nbm.1728
Devaprakash D, Obst SJ, Lloyd DG et al (2020) The free achilles tendon is shorter, stiffer, has larger cross-sectional area and longer T2* relaxation time in trained middle-distance runners compared to healthy controls. Front Physiol 11:965. https://doi.org/10.3389/fphys.2020.00965
Du J, Pak BC, Znamirowski R et al (2009) Magic angle effect in magnetic resonance imaging of the Achilles tendon and enthesis. Magn Reson Imaging 27:557–564. https://doi.org/10.1016/j.mri.2008.09.003
Hansen M, Kjaer M (2016) Sex hormones and tendon. Adv Exp Med Biol 920:139–149. https://doi.org/10.1007/978-3-319-33943-6_13
Funding
No funding to declare.
Author information
Authors and Affiliations
Contributions
DG: manuscript writing/editing. TW: data collection. DR: data collection/manuscript writing. CM: manuscript review. PD: manuscript review. AO: data analysis. CC: data analysis. JPM: manuscript review. LP: data analysis. MG: management. MO: manuscript. review/management. DB: protocol/project development/management. SG: protocol/project development/management.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interest.
Ethical approval
The study was approved by the local Ethic committee (registration number: 2016-A00427-44).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guenoun, D., Wirth, T., Roche, D. et al. Ultra-high field magnetic resonance imaging of the quadriceps tendon enthesis in healthy subjects. Surg Radiol Anat 45, 1049–1054 (2023). https://doi.org/10.1007/s00276-023-03175-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-023-03175-y