Abstract
Background
Endoleaks represent the most common complication after EVAR. Some types are associated with ongoing risk of aneurysm rupture and necessitate long-term surveillance and secondary interventions.
Purpose
This document, as with all CIRSE Standards of Practice documents, will recommend a reasonable approach to best practices of managing endoleaks. This will include imaging diagnosis, surveillance, indications for intervention, endovascular treatments and their outcomes. Our purpose is to provide recommendations based on up-to-date evidence, updating the guidelines previously published on this topic in 2013.
Methods
The writing group was established by the CIRSE Standards of Practice Committee and consisted of clinicians with internationally recognised expertise in endoleak management. The writing group reviewed the existing literature performing a pragmatic evidence search using PubMed to select publications in English and relating to human subjects up to 2023. The final recommendations were formulated through consensus.
Results
Endoleaks may compromise durability of the aortic repair, and long-term imaging surveillance is necessary for early detection and correct classification to guide potential re-intervention. The majority of endoleaks that require treatment can be managed using endovascular techniques. This Standards of Practice document provides up-to-date recommendations for the safe management of endoleaks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The CIRSE Standards of Practice Committee established a writing group, which was tasked with producing up-to-date recommendations for the management of endoleaks following aortic endovascular aneurysm repair (EVAR). CIRSE Standards of Practice documents are not clinical practice guidelines or systematic reviews of the literature; they are not intended to impose a standard of clinical patient care but to recommend a reasonable approach to and best practices for performing endoleak repair. Institutions should regularly review their internal procedures for development and improvement, taking into account international guidance, local resources and regular internal morbidity and mortality reviews.
Methods
The writing group, which was established by the CIRSE Standards of Practice Committee, consisted of six clinicians with internationally recognised expertise in the management of endoleaks. The writing group reviewed existing literature on endoleak repair, performing an extensive evidence review to search for relevant publications in the English language from 1998 to date. Evidence reviewed included guidelines, trials, systematic reviews, and registries, taking into account data on novel techniques, devices, and long-term outcomes that have emerged over the last decade. The writing group formulated the recommendations during three teleconferences and one in-person meeting at the CIRSE Annual Congress 2022.
Background
An endoleak (EL) is defined as persistent blood flow in the aneurysm sac outside the stent graft after aortic endovascular aneurysm repair (EVAR). They represent the most common complication after EVAR with an incidence of 10–50% [1]. Some types are associated with ongoing risk of aneurysm rupture, necessitating long-term surveillance and secondary interventions [2,3,4].
ELs may be classified into primary (present at the time of repair or within 30 days of EVAR) or secondary (occurring after previous negative imaging or beyond 30 days). There are five types of EL based on their anatomical site and aetiology (Table 1). Management depends on EL type and the associated risk of sac rupture [1, 4,5,6,7,8,9,10,11].
The EUROSTAR registry demonstrated that type I and III ELs increase the risk of aneurysm rupture (Table 2) and subsequent studies have identified type I and III ELs to most commonly associated with late rupture, responsible for over 60% of sac ruptures [12, 13] (Table 3). Furthermore, a review of EVAR 1 and 2 trial data showed type II EL with sac expansion to be a risk factor for late aneurysm rupture [14].
Therefore, early detection and correct classification of ELs is vital to plan optimal management. Once detected, ELs that require treatment are managed predominantly with endovascular techniques.
Imaging of Endoleaks
Imaging surveillance is necessary in all patients who undergo EVAR to identify complications including EL, aneurysm sac growth and stent graft migration. The rationale for regular imaging surveillance in the first 5 years after EVAR reflects the significant incidence of complications in this postoperative period [10]. Many centres continue lifelong surveillance, while recognising that this increases the cost of aortic repair by 50% and results in a higher radiation burden for patients [1].
A typical surveillance protocol includes a computed tomography (CT) scan at 1 month after EVAR and at 12 months. Further surveillance with duplex ultrasound (US) rather than CT is considered safe if the CT imaging at 1 year shows no EL and stable sac size, or a type II EL with stable sac size. However, an additional CT at 6 months should be considered if the 1-month scan shows a type I or III EL or (unexplained) aneurysm sac expansion [10, 15,16,17]. Also, detection of a new EL or of aneurysm sac growth of over 5-10 mm on an annual duplex US should prompt further evaluation with CT [1, 10, 16, 18].
Although the mainstay of imaging follow-up relies on CT and US, recent studies and guidelines have highlighted the role of contrast-enhanced US (CEUS) and MR angiography (MRA) in EL surveillance [10, 15, 19, 20]. The combined approach of US, CT and MR can detect up to 91% of ELs [15]. Factors such as patient habitus, EL type, and the local costs and availability of imaging modalities all play a role in deciding optimal imaging follow-up protocols.
A suggested surveillance protocol is outlined in Fig. 1 based on current recommendations.
Computed Tomography Angiography (CTA)
CTA remains the imaging modality of choice in EVAR surveillance and the mainstay of many surveillance protocols. The main drawback of CT is the cumulative radiation dose, which may be significant over lifelong surveillance. A single-phase arterial CT study may be sufficient to depict ELs for standard EVAR follow-up, with delayed phase + / − pre-contrast imaging reserved for problem-solving [1, 11]. It is also important to monitor renal function prior to administration of iodinated contrast to minimise the risk of contrast-induced nephropathy.
Alternatively, advanced CT protocols may be adopted that can generate more than one phase of imaging from a single acquisition, thereby optimising EL visualisation while minimising radiation exposure. Dual-energy CT allows post-processing of CTA datasets to create virtual non-contrast images while reducing beam-hardening artefact from stent graft struts and embolic agents [21]. The split-bolus technique involves intravenous contrast injection in two sequential boluses separated by a time delay, which captures both arterial and venous phase images in a single acquisition. The two protocols may be combined and the resulting dual-energy CTA with split-bolus technique would generate triple phase images from a single acquisition [22].
Colour Duplex Ultrasound (CDUS) and Contrast-Enhanced US (CEUS)
Avoidance of ionising radiation and potentially nephrotoxic contrast agents are the main advantages of CDUS compared with CTA. CDUS also provides dynamic information of ELs, such as flow velocity and direction within the aneurysm sac. Both CDUS and CEUS are accurate in detecting type I and type III ELs as well as sac enlargement [18, 23,24,25], and CEUS has been shown to increase the sensitivity and specificity of CDUS [15, 26, 27]. Several recent studies have suggested CEUS is as sensitive as CTA in detecting ELs and has higher sensitivity for detection of delayed type II ELs [24, 28]. It remains common practice in many institutions to use CDUS in combination with plain films as the mainstay of imaging surveillance.
CDUS and CEUS may be considered as an alternative to CT in stable aneurysms but only in suitable patients with normal body habitus and minimal bowel gas [1, 10, 29]. Other limitations of US include inter-operator variability and an inability to assess the stent graft for migration, seal zones and integrity.
CEUS is often used in conjunction with CTA to further characterise and assess the flow dynamics of a confirmed EL with US contrast. It is also useful in cases of sac expansion with a negative CTA where the contrast may demonstrate sac reperfusion from a slow type II EL [1].
Magnetic Resonance Imaging (MRI)
MRI is used less commonly for EL detection after EVAR. Although the sensitivity of gadolinium-enhanced MRI may be superior to CTA for detection of type II and occult ELs [20, 30, 31], image quality and interpretation are hampered by susceptibility artefact from metallic stent grafts and other sources of metal such as embolisation coils and surgical clips [11, 32, 33]. Nitinol stent grafts are generally MR-compatible as they contain titanium, but the nickel component may still cause some imaging artefact. Many commonly used stent grafts contain MR-incompatible metals including stainless steel, cobalt chromium and elgiloy. MRI should be avoided in these cases as the degree of artefact would render the images non-diagnostic. Further limitations to widespread application of MRI in routine follow-up protocols are prolonged examination time and restricted availability due to high costs [11].
Given the greater sensitivity of MRI for type II and occult ELs and considering the limits of MRI, there is a potential role for MRI in cases of sac expansion without an obvious EL on CTA [31, 33]. Blood pool contrast agents, also known as intravascular contrast agents, remain in the bloodstream for a longer period, increase the signal-to-noise ratio, and ultimately improve image resolution. MRI with these agents may increase the positive yield of slow-flow or occult ELs [33, 34].
Digital Subtraction Angiography (DSA)
Conventional catheter angiography with or without cone-beam CT may be used as a problem-solving tool to determine the type and source of an already detected EL on CTA [1, 11]. For example, type I ELs occurring at the seal zones may be confused with type II EL. Aortography performed with the diagnostic catheter placed above and within the stent graft allows exclusion of a type I EL. Additionally, selective angiography of the superior mesenteric artery (SMA) and iliolumbar arteries allows delineation of the collateral pathways to inferior mesenteric artery (IMA) or lumbar arteries, respectively, as a prelude to embolisation of the type II EL at the same session if suitable. Rotational angiography (catheter angiography with cone-beam CT) may also be useful in cases of sac size increase without a visible EL on non-invasive imaging, although these cases are often challenging to diagnose [11].
Plain Radiography
Plain radiographs in anteroposterior and lateral projections were previously used routinely to assess for migration, stent fractures and modular separations that may result in type I or III ELs. However, as they do not image ELs directly and are limited in the detection of other complications, they are not suitable as the sole imaging modality for surveillance. Many centres no longer use plain radiographs in their follow-up imaging protocols [1, 9].
Type I Endoleak
Definition
Type I EL is defined as a leak at the attachment site of a stent graft, and a manifestation of sealing failure. Type I ELs are further classified into type IA, IB, and IC depending on the occurrence at the proximal or distal ends of the endograft, or iliac occluder, respectively. Type I EL has been reported to occur in as many as 10% of EVAR cases [2, 35,36,37,38,39,40] and appear to increase with time from less than 5% incidence on surveillance imaging at 30 days to 6.8% incidence at 12 months [38].
Type IA EL is associated with adverse proximal neck anatomy pre-EVAR, including short (> 15 mm), angulated (> 60°), large diameter (> 32 mm), conical or tapered necks or those with calcification or thrombus [1, 10, 41,42,43,44]. Patients with hostile neck anatomy often require adjunctive procedures to achieve an adequate proximal seal and are associated with a four-fold increased risk of developing type IA EL [45]. The use of EVAR in challenging necks outside the device instructions for use (IFU) is associated with a higher incidence of type IA EL, which may contribute to delayed rupture and poor outcomes [46]. In view of this, such practice is discouraged.
Type IB EL is associated with large diameter common iliac arteries > 14 mm, short iliac sealing zones and iliac artery tortuosity [47, 48]. Around 50% occur within 6 months after EVAR and iliac artery expansion at the landing zones in the early postoperative months may result in a loss of seal.
Indication for Treatment
Type I EL is associated with elevated sac pressure and an ongoing risk of aneurysm expansion and rupture [2, 3, 12, 14, 49,50,51]. It is the leading cause of late aneurysm rupture in up to 52% of cases [13] and should be treated promptly upon detection [1, 9,10,11].
Management
Primary Type IA
Intraprocedural type IA EL can be treated with repeated balloon moulding or placement of a giant bare metallic stent at the proximal neck. If additional landing zone length exists, a short aortic cuff may be used to extend the seal zone.
EndoAnchors (Aptus Heli-FX EndoAnchor System, Medtronic, USA) staple the stent graft to the aortic wall by means of a metallic tack. They may be used as an adjunct to prevent type IA EL and stent graft migration in challenging aortic necks or to treat a visible EL [52]. The only comparable controlled data did not show a significant difference in the rate of type 1A EL between stapled and non-stapled cases [53].
Secondary Type IA
Delayed type IA EL may occur due to changes in the configuration of the aorta that result in aortic neck dilatation or stent graft migration. Multiple options for re-intervention are available including balloon moulding of the proximal seal zone and placement of bare stents to increase the radial strength at the proximal attachment site.
In selected patients with a suitable landing zone, proximal extension of the stent graft may be considered by means of a simple cuff, either alone or in combination with parallel chimney grafts. Patients with more challenging anatomy may require proximal extension using custom-made fenestrated or branched devices.
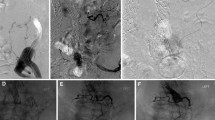
In patients where these techniques have failed, where there is insufficient neck length for stent graft extension, or where the patient is unfit for more complex therapies, transcatheter embolisation is an alternative EL treatment option [1, 10, 11] (Fig. 2). The entry channel between the aortic wall and stent graft is engaged with a guide catheter from a femoral, brachial or radial approach. A microcatheter is introduced coaxially into the EL cavity and an angiogram is performed to assess the size and extent of the EL cavity, the size of the entry channel (neck), and any exiting vessels. Embolisation can be performed with endovascular coils, liquid embolics such as n-butyl cyanoacrylate (NBCA), or ethylene vinyl alcohol copolymer (EVOH) (Onyx, Medtronic, USA), thrombin or a combination of embolic agents.
Type IA EL embolisation. (A) Sagittal CT image shows proximal type I EL post-EVAR (arrow). Unsuitable for treatment with an aortic cuff due to heavily diseased iliac arteries. (B) Entry channel between aortic wall and stent graft was engaged with a reverse-curve catheter. The subsequent angiogram outlines a large EL cavity (arrows). (C) Embolisation of EL cavity with coils via a microcatheter. (D) Completion angiogram shows successful embolisation
When endovascular techniques have failed to control a type IA EL, conversion to an open surgical approach may be the only option [1, 9].
Type IA in Chimney-EVAR (Ch-EVAR)
Primary type IA EL is common after Ch-EVAR occurring in up to 30% of patients, but the majority resolve spontaneously by 12 months and they are not associated with aneurysm sac growth [54]. About 3% require re-intervention for persistent EL and these pose a challenge as standard treatment options to optimise the proximal seal may not be feasible. If simple endovascular techniques such as simultaneous ballooning of the stent graft and chimneys do not control the EL, embolisation techniques should be considered [11].
Type IB
Type IB EL is treated with distal extension of the iliac limb to achieve an adequate distal seal (Fig. 3). If there is insufficient length before the origin of the internal iliac artery, it may be necessary to extend the stent graft into the external iliac artery. The internal iliac artery (IIA) can be over stented, embolised with coils or plugs, or preserved with an iliac branch device or parallel grafts [10, 11, 29].
Type IC
EVAR with an aorto-uni-iliac (AUI) device is much less commonly performed than previously and type IC EL is rarely reported. Treatment involves placing additional plugs or coils to achieve complete occlusion of the contralateral common iliac artery.
Type II Endoleak
Definition
Type II ELs are caused by retrograde blood flow into the aneurysm sac from aortic or iliac branch arteries, such as lumbar, inferior mesenteric (IMA), accessory renal, median sacral and internal iliac arteries [1, 55]. They are further classified into type IIA EL when only one branch is involved, and type IIB EL when there are two or more branches, usually with a dominant inflow artery and one or more outflow arteries [11]. Type II ELs are the most common EL following EVAR and a major cause for re-intervention. They occur in up to 20–30% of cases [2, 38, 39, 56,57,58]. Approximately 50% resolve spontaneously in the first 6 months, 5–10% persist beyond 6 months and new type II ELs develop in 5–10% [11, 56, 59, 60] of patients. Factors that increase the risk of developing type II EL include a patent IMA > 3 mm in diameter, a lumbar artery > 2 mm, an aorto-iliac aneurysm and/or a significant mural thrombus burden [57, 61,62,63,64,65,66].
Indication for Treatment
There is debate regarding the clinical significance of type II EL and the threshold for intervention. Some authors suggest a more conservative approach as the ELs are inherently low-flow and often transient. The risk of aneurysm rupture in the presence of an isolated type II EL has been shown to be less than 1% [56] and type II EL has not been associated with reduced patient survival [2]. Similarly, several studies have found no differences in aneurysm-related mortality or sac expansion between patients who were treated conservatively for type II EL and those who underwent re-intervention [67, 68].
However, other authors have presented contradictory findings and have demonstrated that some type II ELs may not be benign and are associated with adverse outcomes. For example, type II EL has been shown to be an independent risk factor for sac expansion [69,70,71] and persistent type II EL to be associated with a higher incidence of re-intervention, rupture and conversion to open surgery [57, 69].
The current consensus is to consider intervention in persistent type II ELs when they are associated with significant sac expansion. This is commonly considered to be > 5 – 10 mm [1, 9,10,11, 55, 56] over 12 months [11, 55]. Type II EL with stable sac size should be managed conservatively with regular imaging follow-up as proposed in Fig. 1.
Management
In suspected cases of type II EL associated with sac expansion, it is important to consider whether this could represent an occult type I or III masquerading as a type II. If CTA is inconclusive, catheter angiography + / − cone-beam CT may be useful to clarify the source of the EL and plan endovascular treatment, which may be performed at the same time [11].
The mainstay of treatment of type II EL is embolisation with the aim of occluding the arteries supplying the EL as well as the EL cavity itself [11, 55]. Complex type II EL with multiple supplying arteries may behave in a similar manner to a high-flow vascular malformation with a central nidus and multiple inflow and outflow vessels [11]. The choice of intervention approach and technique are dependent on the anatomy of the EL and operator experience. In some challenging cases, more than one technique may be used to achieve successful embolisation. CTA with multiplanar reformats is useful in case planning and pre-empting potential technical challenges.
Transarterial [55, 66, 72,73,74,75,76,77,78,79,80,81]
Transarterial embolisation is the most common technique that involves catheterization of the dominant feeding vessel via collateral channels. This approach is most successful in ELs involving the IMA where there is usually a long and tortuous course but one that is relatively large in calibre. Conversely, technical success may be limited in lumbar ELs where the feeding artery can be remote from the iliolumbar artery via small and tortuous branches.
Type II EL involving the IMA may be accessed via the SMA. The usual route is as follows: SMA—middle colic artery—arc of Riolan or marginal artery of Drummond (which is usually hypertrophied)—left colic artery—IMA—aneurysm sac—EL cavity (Fig. 4). The SMA and middle colic artery are selected in turn with a 4- or 5-Fr catheter supported by a long vascular sheath. A microcatheter is then coaxially advanced along the long and tortuous route to the IMA and into the EL cavity. An angiogram is performed to outline the EL cavity and any outflow arteries, and to assess the overall flow. Embolisation is commonly performed with liquid embolic agents (e.g. NBCA, EVOH) especially when there is a large EL cavity to fill. In high-flow ELs that involve multiple arteries, metallic coils may be used to prevent inadvertent non-target distal embolisation of the liquid embolic agent. This can be achieved by embolising one or more outflow arteries or loosely packing the EL cavity with coils, which reduces the overall blood flow and therefore the degree of distal penetration of the liquid agent.
Transarterial embolisation of type II EL. (A) Axial CT image shows type II EL in the anterior sac (arrow) close to the IMA (arrowhead). (B) Angiogram from the middle colic branch of the SMA opacifies an hypertrophied arc of Riolan, IMA and EL cavity (arrowhead). (C) Embolisation with liquid embolic agent via a microcatheter. (D) Completion angiogram shows no further EL opacification
Type II EL involving lumbar arteries may be accessed via the iliolumbar artery from an ipsilateral common femoral access. Once the optimal course has been outlined on angiography, a microcatheter is advanced coaxially into the feeding lumbar artery and into the EL nidus. Angiography from the EL nidus may opacify other lumbar arteries that are involved which should also be embolised. Similarly, if there are multiple and fast-flowing arteries with a large nidus, it may be prudent to coil embolise these branches first before filling the nidus with a liquid embolic agent. If it is not possible to reach the nidus from this approach, a more proximal embolisation may be attempted either with a lower viscosity liquid agent or with coils [55]. However, this may result in recurrence of the EL from collateral vessels at other lumbar levels.
Direct Sac Puncture [82,83,84]
The EL nidus may be targeted directly by percutaneous puncture of the aneurysm sac. This may be performed via a transabdominal approach with the patient supine or a translumbar approach with the patient positioned prone. CTA and on-table Doppler US are used to plan a safe percutaneous route to the EL cavity avoiding bowel or large vessels and a trocar needle (18–20G) is advanced through the aneurysm sac into the EL cavity under sonographic guidance. Satisfactory position within the EL cavity is confirmed when arterial flow blood is seen from the hub of the needle and angiography is then performed to opacify the EL cavity and feeding arteries. Embolisation with a liquid embolic may be performed directly through the outer cannula of the trocar device. Alternatively, the trocar cannula may be exchanged for a 4-Fr sheath over a stiff guidewire. Large outflow vessels may be embolised with coils prior to embolisation of the nidus with a liquid agent. It may be necessary to use a microcatheter and guidewire deeper into the nidus and/or feeding vessels prior to embolisation.
Transiliac Paraendograft [85,86,87]
This is an adjunctive technique when the transarterial technique has been unsuccessful in reaching the EL. It may be possible to manipulate a catheter and hydrophilic guidewire into the potential space between the iliac limb endograft and arterial wall, navigating through the sac thrombus into the EL cavity. Once the position of the catheter tip within the EL cavity is confirmed on angiography, the EL is embolised with a liquid embolic agent ± coils as appropriate.
Transcaval [88,89,90,91]
This adjunctive technique involves accessing the aneurysm sac via percutaneous puncture of the inferior vena cava (IVC). With the patient positioned prone, an angled sheathed needle from a transjugular liver access set (e.g. Colapinto, Angiodynamics, USA; Rosch-Uchida, Cook, USA) is used to pierce the wall of the IVC into the adjacent aneurysm sac to reach the EL cavity. A catheter is then advanced through the sheath for subsequent embolisation. This approach may be beneficial in a small number of patients where the EL cavity is located on the right side of the aneurysm sac that is not amenable to access by other techniques.
Surgical
Surgical treatment options include laparoscopic clipping of aortic side branches, open surgical ligation of bleeding vessels and sac plication. Surgery is usually reserved for cases where endovascular techniques have been unsuccessful.
Type III Endoleak
Definition
Type III ELs arise from a structural defect of the stent graft, either secondary to a modular disconnection of its components (IIIA), a fabric tear (IIIB) or a junctional separation of fenestrated or branched stent grafts (IIIC). It may occur due to inadequate overlap of stent graft components, device migration or material fatigue.
Type III EL is relatively uncommon with a reported incidence of 0.7–4.5% [2, 4, 36, 38, 92]. First- and second-generation stent grafts are associated with a significantly higher incidence of type III EL when compared with more recent third generation devices, 12.7% and 1.2%, respectively [92]. Most fabric failures have been found to be associated with specific graft materials and designs, which have subsequently been modified or withdrawn from the market. A recent example is the earlier generation of the AFX device (Endologix, USA) that was withdrawn in 2016 after unacceptable rates of type III EL were reported and the US Food and Drug Administration intervened [93, 94].
The reported incidence of type III EL in fenestrated or branched EVAR is more variable. A higher degree of device modularity and procedural complexity does not appear to increase the incidence of type III EL as demonstrated in a large multicentre retrospective cohort study of over 4,000 cases. Type III EL remained relatively uncommon at 4% and the majority were primary ELs identified around the time of the index procedure [95]. This contrasts with the findings of single-centre series of complex EVAR where type III EL was seen in as many as 12% of patients, the majority of which were secondary EL, and type III EL was identified as the most frequent indication for re-intervention. Fenestrated EVAR (F-EVAR) with large diameter devices (34-36 mm) appear to have an increased risk of type III EL and the need for re-intervention [96].
Indication for Treatment
Type III EL leads to increased pressure within the aneurysm sac and are associated with a risk of aneurysm rupture. They therefore warrant prompt treatment once detected [1, 9,10,11, 97, 98].
Management
Treatment of a type III EL may include endovascular, hybrid and open surgical techniques. Intraoperative type III ELs should be treated at the time of diagnosis, which can often be achieved by repeat balloon dilatation of areas of component overlap or by placing an additional stent graft across the separated components to bridge the gap. Similarly, the mainstay of treating secondary type IIIA or IIIC EL is by placing a bridging stent graft or aortic cuff to close the gap between the separated components [1, 92,93,94,95,96,97,98,99,100,101] (Fig. 5).
Type III EL in a F-EVAR. (A) Axial CT image shows junctional separation between the main body and fenestrated left renal stent (arrowhead) resulting in type III EL (arrow). (B) Fluoroscopic image confirms a visible gap between the two stent components (arrow heads). (C) Additional renal stent graft deployed to bridge the gap. (D) Angiogram shows no residual EL
Type IIIB EL from a fabric tear may be treated by relining the main body or iliac limb. However, if the tear lies close to the flow divider, treatment becomes more complex. It may be possible to place a new bifurcated device within the existing device if there is sufficient length between the proximal landing zone and the flow divider of the existing main body to enable correct deployment of the contralateral limb of the new device. If this is not feasible, a custom-made device with an inverted iliac limb may be considered if one is available. A hybrid solution of placing an AUI device with contralateral common iliac occlusion and a surgical femoral-femoral bypass is often used in cases where there is no suitable endovascular option [101]. Finally, conversion to open surgery may be considered if these techniques fail to treat the EL [1, 9, 92, 98].
Type IV Endoleak
Type IV EL represents leakage of blood through the stent graft due to fabric porosity in the early postoperative period. They were described mainly in first-generation stent grafts at the time of completion angiography when patients are fully anticoagulated. They are rare in newer generation devices, are self-limiting and do not require re-intervention [1, 9, 11, 12].
Type V Endoleak
Definition
Type V EL is also known as endotension and represents aneurysm sac expansion in the absence of an identifiable EL. This may be due to such slow blood flow that it is below the sensitivity limits for detection on current imaging methods [102,103,104,105]. For example, several authors have suggested that the vasa vasorum of the aneurysmal aortic wall may be a source of occult type II EL [106,107,108]. Other alternative hypotheses include hyperfibrinolysis and local coagulation activation leading to sac hygromas [109,110,111], ultrafiltration across the fabric of the endograft [112, 113], and pressure transmission through thrombus or the stent graft [102, 103]. It is an uncommon phenomenon reported in 2–3% [114] of EVARs and as the underlying mechanism remains contentious, each case is treated on an individual basis. Observation may be appropriate in some cases but the criteria for conservative management are unclear [2].
Indication for Treatment
Type V EL is a diagnosis of exclusion when all other causes of sac expansion have excluded. Potential cases require additional imaging to exclude an occult EL. MRI has been shown to be more sensitive than CTA for the detection of type II EL and some authors recommend its use in patients with a suspected type V EL [31]. Catheter angiography with cone-beam CT has also been suggested as a useful imaging adjunct [11].
Management
Treatment of type V EL is not yet defined and remains a challenge. Reported interventions include percutaneous sac aspiration, open surgical exploration and sac plication/resection, which have been unsuccessful in preventing sac enlargement [109, 110, 115]. Relining of the entire stent graft has also been described [111]. If device relining fails, then open surgical conversion may be the only viable option.
Outcomes
Primary Type IA
Repeated balloon moulding, giant bare metallic stents and short aortic cuff are successful in 90–100% [116,117,118].
EndoAnchors may be useful in challenging aortic necks but outcomes to date have not demonstrated significant benefit in reducing type IA EL when compared with non-stapled cases using latest generation of stent grafts [52, 53].
Secondary Type IA
Early outcomes of various treatment strategies are outlined in Table 4. The choice of treatment should be based on the patient's condition, the characteristics of endoleak and the anatomy of the aorta. As suggested in a recent meta-analysis single or double chimney grafts can be an alternative to simple or fenestrated cuffs [119]. EndoAnchors have no benefit over conservative management in secondary type IA EL [119].
Transcatheter embolisation is successful in the short-term but endoleak recurrence is highly variable, between 0 and 58% at 2 years [119,120,121,122,123,124,125,126,127,128,129]. Therefore, embolisation should be considered in a select cohort of patients where traditional endovascular and surgical options are unsuitable or have failed.
Type IB
Distal stent graft extension is successful in 90–100% of cases with less than 7% requiring endovascular re-intervention [47, 130]. Sacrificing the IIA can result in buttock claudication in up to 6.7% of cases, although symptoms are often transient or improve with time [130].
Type II
There is wide variation in the reported technical and clinical success rates of type II EL embolisation [55, 72, 131, 132] from mostly retrospective single-centre series with relatively small numbers [73,74,75,76, 82,83,84,85,86,87,88,89,90,91, 108, 133,134,135]. Most studies report promising technical success rates of 80–100% but there is variation in how clinical outcome is defined and reported.
One of the largest series with 5-year outcome data showed transarterial embolisation with glue or coils can maintain stable sac size in 82% at 1 year but this dropped to 44% by 5 years [131]. Embolisation with coils only were more likely to require a second intervention [131] and embolisation of IMA EL (72%) yield better outcomes than lumbar EL (17%) at 2 years [77], and 7 patients experienced continued sac expansion and required conversion to open repair [77].
Despite initial success, there is a significant EL recurrence rate and delayed sac expansion. Some authors have suggested that failure to occlude the EL cavity or nidus may explain late failure rates after transarterial coil embolisation of the feeding vessel only [11, 66, 76, 136, 137].
Major complications associated with type II EL embolisation are rare but complications arising from non-target embolisation have been reported. These include pulmonary embolus secondary to glue escape via the inferior vena cava, mesenteric ischaemia secondary to IMA embolisation, lumbar radiculopathy from distal embolisation of lumbar arteries, and debilitating acute lower limb claudication [74, 78, 82, 131, 137].
Type III
A large retrospective series identified 20 patients with type III EL, all of whom underwent endovascular treatment. Four patients (20%) suffered major periprocedural complications including lower limb ischaemia, retroperitoneal haematoma and bowel ischaemia. In addition, during a mean follow-up of 10.6 years, additional intervention was necessary in five patients (25%) for recurrent type III EL and three patients required conversion to open repair [92].
Conclusion
ELs are common complications after EVAR and may compromise the durability of aortic repair. Long-term imaging surveillance is necessary for early detection and correct classification of ELs to guide potential re-intervention. Understanding the risk factors for ELs is important for the prevention of potential ELs, which is aided by the development of new techniques and improved stent graft designs. The majority of ELs that require treatment can be managed using endovascular techniques. A clear understanding of the EL type, and therefore its likely aetiology, is essential to guide decision-making around intervention.
Abbreviations
- EL:
-
Endoleaks
- EVAR:
-
Endovascular aneurysm repair
- CT:
-
Computed tomography
- US:
-
Ultrasound
- CEUS:
-
Contrast-enhanced ultrasound
- MRA:
-
Magnetic resonance angiography
- BMI:
-
Body mass index
- CDUS:
-
Colour duplex ultrasound
- IFU:
-
Instructions for use
- NBCA:
-
N-butyl cyanoacrylate
- EVOH:
-
Ethylene vinyl alcohol
- Ch-EVAR:
-
Chimney endovascular aneurysm repair
- AUI:
-
Aorto-uni-iliac device
- IMA:
-
Inferior mesenteric artery
- SMA:
-
Superior mesenteric artery
- IVC:
-
Inferior vena cava
- F-EVAR:
-
Fenestrated endovascular aneurysm repair
References
Rand T, Uberoi R, Cil B, Munneke G, Tsetis D. Quality improvement guidelines for imaging detection and treatment of endoleaks following endovascular aneurysm repair (EVAR). Cardiovasc Intervent Radiol. 2013;36(1):35–45.
Powell JT, Sweeting MJ, Ulug P, Blankensteijn JD, Lederle FA, Becquemin JP, Greenhalgh RM; EVAR-1, DREAM, OVER and ACE Trialists. Meta-analysis of individual-patient data from EVAR-1, DREAM, OVER and ACE trials comparing outcomes of endovascular or open repair for abdominal aortic aneurysm over 5 years. Br J Surg. 2017;104(3):166–178.
Patel R, Sweeting MJ, Powell JT, Greenhalgh RM; EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388(10058):2366–2374.
van Marrewijk C, Buth J, Harris PL, Norgren L, Nevelsteen A, Wyatt MG. Significance of endoleaks after endovascular repair of abdominal aortic aneurysms: The EUROSTAR experience. J Vasc Surg. 2002;35(3):461–73.
White GH, Yu W, May J, Chaufour X, Stephen MS. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. J Endovasc Surg. 1997;4(2):152–68.
Rosen RJ, Green RM. Endoleak management following endovascular aneurysm repair. J Vasc Interv Radiol. 2008;19(6 Suppl):S37-43.
Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, van Herwaarden JA, Holt PJ, van Keulen JW, Rantner B, Schlösser FJ, Setacci F, Ricco JB; European Society for Vascular Surgery. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 Suppl 1:S1-S58.
Baum RA, Stavropoulos SW, Fairman RM, Carpenter JP. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2003;14(9 Pt 1):1111–7.
Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, Dick F, van Herwaarden J, Karkos C, Koelemay M, Kölbel T, Loftus I, Mani K, Melissano G, Powell J, Szeberin Z, Esvs Guidelines Committee, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Kolh P, Lindholt JS, de Vega M, Vermassen F, Document Reviewers, Björck M, Cheng S, Dalman R, Davidovic L, Donas K, Earnshaw J, Eckstein HH, Golledge J, Haulon S, Mastracci T, Naylor R, Ricco JB, Verhagen H. European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8–93.
Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML, Starnes BW. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2-77.e2.
Ameli-Renani S, Pavlidis V, Morgan RA. Secondary endoleak management following TEVAR and EVAR. Cardiovasc Intervent Radiol. 2020;43(12):1839–54.
Schlösser FJ, Gusberg RJ, Dardik A, Lin PH, Verhagen HJ, Moll FL, Muhs BE. Aneurysm rupture after EVAR: can the ultimate failure be predicted? Eur J Vasc Endovasc Surg. 2009;37(1):15–22.
Antoniou GA, Georgiadis GS, Antoniou SA, Neequaye S, Brennan JA, Torella F, Vallabhaneni SR. Late rupture of abdominal aortic aneurysm after previous endovascular repair: a systematic review and meta-analysis. J Endovasc Ther. 2015;22(5):734–44.
Wyss TR, Brown LC, Powell JT, Greenhalgh RM. Rate and predictability of graft rupture after endovascular and open abdominal aortic aneurysm repair: data from the EVAR Trials. Ann Surg. 2010;252(5):805–12.
Zaiem F, Almasri J, Tello M, Prokop LJ, Chaikof EL, Murad MH. A systematic review of surveillance after endovascular aortic repair. J Vasc Surg. 2018;67(1):320-331.e37.
Sternbergh WC, Greenberg RK, Chuter TA, Tonnessen BH; Zenith Investigators. Redefining postoperative surveillance after endovascular aneurysm repair: recommendations based on 5-year follow-up in the US Zenith multicenter trial. J Vasc Surg. 2008;48(2):278–84.
Go MR, Barbato JE, Rhee RY, Makaroun MS. What is the clinical utility of a 6-month computed tomography in the follow-up of endovascular aneurysm repair patients? J Vasc Surg. 2008;47(6):1181–6.
Nyheim T, Staxrud LE, Rosen L, Slagsvold CE, Sandbaek G, Jørgensen JJ. Review of postoperative CT and ultrasound for endovascular aneurysm repair using Talent stent graft: can we simplify the surveillance protocol and reduce the number of CT scans? Acta Radiol. 2013;54(1):54–8.
Abdominal aortic aneurysm: diagnosis and management. London: National Institute for Health and Care Excellence (NICE); 2020 Mar 19. Available at: https://www.nice.org.uk/guidance/ng156
Cassagnes L, Pérignon R, Amokrane F, Petermann A, Bécaud T, Saint-Lebes B, Chabrot P, Rousseau H, Boyer L. Aortic stent-grafts: endoleak surveillance. Diagn Interv Imaging. 2016;97(1):19–27.
Flors L, Leiva-Salinas C, Norton PT, Patrie JT, Hagspiel KD. Endoleak detection after endovascular repair of thoracic aortic aneurysm using dual-source dual-energy CT: suitable scanning protocols and potential radiation dose reduction. AJR Am J Roentgenol. 2013;200(2):451–60.
Javor D, Wressnegger A, Unterhumer S, Kollndorfer K, Nolz R, Beitzke D, Loewe C. Endoleak detection using single-acquisition split-bolus dual-energy computer tomography (DECT). Eur Radiol. 2017;27(4):1622–30.
Chaer RA, Gushchin A, Rhee R, Marone L, Cho JS, Leers S, Makaroun MS. Duplex ultrasound as the sole long-term surveillance method post-endovascular aneurysm repair: a safe alternative for stable aneurysms. J Vasc Surg. 2009;49(4):845–9.
Chung J, Kordzadeh A, Prionidis I, Panayiotopoulos Y, Browne T. Contrast-enhanced ultrasound (CEUS) versus computed tomography angiography (CTA) in detection of endoleaks in post-EVAR patients. Are delayed type II endoleaks being missed? A systematic review and meta-analysis. J Ultrasound. 2015;18(2):91–9.
Zimmermann H, D’Anastasi M, Rjosk-Dendorfer D, Helck A, Meimarakis G, Reiser M, Clevert DA. Value of high-resolution contrast-enhanced ultrasound in detection and characterisation of endoleaks after EVAR. Clin Hemorheol Microcirc. 2014;58(1):247–60.
Mirza TA, Karthikesalingam A, Jackson D, Walsh SR, Holt PJ, Hayes PD, Boyle JR. Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: systematic review and bivariate meta-analysis. Eur J Vasc Endovasc Surg. 2010;39(4):418–28.
Henao EA, Hodge MD, Felkai DD, McCollum CH, Noon GP, Lin PH, Lumsden AB, Bush RL. Contrast-enhanced Duplex surveillance after endovascular abdominal aortic aneurysm repair: improved efficacy using a continuous infusion technique. J Vasc Surg. 2006;43(2):259–64.
Kapetanios D, Kontopodis N, Mavridis D, McWilliams RG, Giannoukas AD, Antoniou GA. Meta-analysis of the accuracy of contrast-enhanced ultrasound for the detection of endoleak after endovascular aneurysm repair. J Vasc Surg. 2019;69(1):280–94.
Cannavale A, Lucatelli P, Corona M, Nardis P, Basilico F, De Rubeis G, Santoni M, Catalano C, Bezzi M. Evolving concepts and management of endoleaks after endovascular aneurysm repair: where do we stand in 2019? Clin Radiol. 2020;75(3):169–78.
Guo Q, Zhao J, Huang B, Yuan D, Yang Y, Zeng G, Xiong F, Du X. A systematic review of ultrasound or magnetic resonance imaging compared with computed tomography for endoleak detection and aneurysm diameter measurement after endovascular aneurysm repair. J Endovasc Ther. 2016;23(6):936–43.
Habets J, Zandvoort HJ, Reitsma JB, Bartels LW, Moll FL, Leiner T, van Herwaarden JA. Magnetic resonance imaging is more sensitive than computed tomography angiography for the detection of endoleaks after endovascular abdominal aortic aneurysm repair: a systematic review. Eur J Vasc Endovasc Surg. 2013;45(4):340–50.
Schwope RB, Alper HJ, Talenfeld AD, Cohen EI, Lookstein RA. MR angiography for patient surveillance after endovascular repair of abdominal aortic aneurysms. AJR Am J Roentgenol. 2007;188(4):W334–40.
Cornelissen SA, Prokop M, Verhagen HJ, Adriaensen ME, Moll FL, Bartels LW. Detection of occult endoleaks after endovascular treatment of abdominal aortic aneurysm using magnetic resonance imaging with a blood pool contrast agent: preliminary observations. Invest Radiol. 2010;45(9):548–53.
Wieners G, Meyer F, Halloul Z, Peters N, Rühl R, Dudeck O, Tautenhahn J, Ricke J, Pech M. Detection of type II endoleak after endovascular aortic repair: comparison between magnetic resonance angiography and blood-pool contrast agent and dual-phase computed tomography angiography. Cardiovasc Intervent Radiol. 2010;33(6):1135–42.
Veith FJ, Baum RA, Ohki T, Amor M, Adiseshiah M, Blankensteijn JD, Buth J, Chuter TA, Fairman RM, Gilling-Smith G, Harris PL, Hodgson KJ, Hopkinson BR, Ivancev K, Katzen BT, Lawrence-Brown M, Meier GH, Malina M, Makaroun MS, Parodi JC, Richter GM, Rubin GD, Stelter WJ, White GH, White RA, Wisselink W, Zarins CK. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35(5):1029–35.
Faries PL, Cadot H, Agarwal G, Kent KC, Hollier LH, Marin ML. Management of endoleak after endovascular aneurysm repair: cuffs, coils, and conversion. J Vasc Surg. 2003;37(6):1155–61.
Mehta M, Sternbach Y, Taggert JB, Kreienberg PB, Roddy SP, Paty PS, Ozsvath KJ, Darling RC 3rd. Long-term outcomes of secondary procedures after endovascular aneurysm repair. J Vasc Surg. 2010;52(6):1442–9.
Drury D, Michaels JA, Jones L, Ayiku L. Systematic review of recent evidence for the safety and efficacy of elective endovascular repair in the management of infrarenal abdominal aortic aneurysm. Br J Surg. 2005;92(8):937–46.
Chisci E, Guidotti A, Pigozzi C, Frosini P, Sapio PL, Troisi N, Ercolini L, Michelagnoli S. Long-term analysis of standard abdominal aortic endovascular repair using different grafts focusing on endoleak onset and its evolution. Int J Cardiol. 2019;1(276):53–60.
Qazi AA, Jaberi A, Mironov O, Addas J, Qazi E, Tarulli E, Simons M, Tan KT. Conservative management of type 1A endoleaks at completion angiogram in endovascular repair of infra-renal abdominal aortic aneurysms with current generation stent grafts. Vascular. 2019;27(2):168–74.
AbuRahma AF, Yacoub M, Mousa AY, Abu-Halimah S, Hass SM, Kazil J, AbuRahma ZT, Srivastava M, Dean LS, Stone PA. Aortic neck anatomic features and predictors of outcomes in endovascular repair of abdominal aortic aneurysms following versus not following instructions for use. J Am Coll Surg. 2016;222(4):579–89.
Naughton PA, Garcia-Toca M, Rodriguez HE, Keeling AN, Resnick SA, Morasch MD, Eskandari MK. Endovascular treatment of delayed type 1 and 3 endoleaks. Cardiovasc Intervent Radiol. 2011;34(4):751–7.
Pitoulias GA, Valdivia AR, Hahtapornsawan S, Torsello G, Pitoulias AG, Austermann M, Gandarias C, Donas KP. Conical neck is strongly associated with proximal failure in standard endovascular aneurysm repair. J Vasc Surg. 2017;66(6):1686–95.
Schuurmann RC, Ouriel K, Muhs BE, Jordan WD Jr, Ouriel RL, Boersen JT, de Vries JP. Aortic curvature as a predictor of intraoperative type Ia endoleak. J Vasc Surg. 2016;63(3):596–602.
Antoniou GA, Georgiadis GS, Antoniou SA, Kuhan G, Murray D. A meta-analysis of outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile and friendly neck anatomy. J Vasc Surg. 2013;57(2):527–38.
Zacharias N, Warner CJ, Taggert JB, Roddy SP, Kreienberg PB, Ozsvath KJ, Sternbach Y, Darling RC 3rd. Anatomic characteristics of abdominal aortic aneurysms presenting with delayed rupture after endovascular aneurysm repair. J Vasc Surg. 2016;64(6):1629–32.
Bianchini Massoni C, Mascoli C, Perini P, Tecchio T, Gallitto E, Azzarone M, Gargiulo M, Freyrie A, Faggioli G, Stella A. Endovascular treatments for type Ib endoleaks after aorto-iliac aneurysms exclusion: mid-term results. Int Angiol. 2018;37(5):384–9.
Coulston J, Baigent A, Selvachandran H, Jones S, Torella F, Fisher R. The impact of endovascular aneurysm repair on aortoiliac tortuosity and its use as a predictor of iliac limb complications. J Vasc Surg. 2014;60(3):585–9.
Schurink GW, Aarts NJ, Wilde J, van Baalen JM, Chuter TA, Schultze Kool LJ, van Bockel JH. Endoleakage after stent-graft treatment of abdominal aneurysm: implications on pressure and imaging–an in vitro study. J Vasc Surg. 1998;28(2):234–41.
Buth J, Laheij RJ. Early complications and endoleaks after endovascular abdominal aortic aneurysm repair: report of a multicenter study. J Vasc Surg. 2000;31(1 Pt 1):134–46.
Harris PL, Dimitri S. Predicting failure of endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 1999;17(1):1–2.
Qamhawi Z, Barge TF, Makris GC, Patel R, Wigham A, Anthony S, Uberoi R. Systematic review of the use of endoanchors in endovascular aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2020;59(5):748–56.
Muhs BE, Jordan W, Ouriel K, Rajaee S, de Vries JP. Matched cohort comparison of endovascular abdominal aortic aneurysm repair with and without EndoAnchors. J Vasc Surg. 2018;67(6):1699–707.
Ullery BW, Tran K, Itoga NK, Dalman RL, Lee JT. Natural history of gutter-related type Ia endoleaks after snorkel/chimney endovascular aneurysm repair. J Vasc Surg. 2017;65(4):981–90.
Chung R, Morgan RA. Type 2 endoleaks post-EVAR: current evidence for rupture risk, intervention and outcomes of treatment. Cardiovasc Intervent Radiol. 2015;38(3):507–22.
Sidloff DA, Gokani V, Stather PW, Choke E, Bown MJ, Sayers RD. Type II endoleak: conservative management is a safe strategy. Eur J Vasc Endovasc Surg. 2014;48(4):391–9.
El Batti S, Cochennec F, Roudot-Thoraval F, Becquemin JP. Type II endoleaks after endovascular repair of abdominal aortic aneurysm are not always a benign condition. J Vasc Surg. 2013;57(5):1291–7.
Haq IU, Kelay A, Davis M, Brookes J, Mastracci TM, Constantinou J. Ten-year single-centre experience with type II endoleaks: Intervention versus observation. Vasc Med. 2017;22(4):316–23.
Lo RC, Buck DB, Herrmann J, Hamdan AD, Wyers M, Patel VI, Fillinger M, Schermerhorn ML (2016) Vascular Study Group of New England. Risk factors and consequences of persistent type II endoleaks. J Vasc Surg 63(4):895–901.
Gelfand D, White G, Wilson S. Clinical significance of type II endoleak after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg. 2006;20(1):69–74.
Manunga JM, Cragg A, Garberich R, Urbach JA, Skeik N, Alexander J, Titus J, Stephenson E, Alden P, Sullivan TM. Preoperative inferior mesenteric artery embolisation: A valid method to reduce the rate of type II endoleak after EVAR? Ann Vasc Surg. 2017;39:40–7.
Guo Q, Du X, Zhao J, Ma Y, Huang B, Yuan D, Yang Y, Zeng G, Xiong F. Prevalence and risk factors of type II endoleaks after endovascular aneurysm repair: A meta-analysis. PLoS ONE. 2017;12(2):e0170600.
Lalys F, Durrmann V, Duménil A, Göksu C, Cardon A, Clochard E, Lucas A, Kaladji A. Systematic review and meta-analysis of preoperative risk factors of type II endoleaks after endovascular aneurysm repair. Ann Vasc Surg. 2017;41:284–93.
Alerci M, Giamboni A, Wyttenbach R, Porretta AP, Antonucci F, Bogen M, Toderi M, Guerra A, Sartori F, Tutta P, Inglese L, Limoni C, Gallino A, Von Segesser LK. Endovascular abdominal aneurysm repair and impact of systematic preoperative embolisation of collateral arteries: endoleak analysis and long-term follow-up. J Endovasc Ther. 2013;20(5):663–71.
Ward TJ, Cohen S, Patel RS, Kim E, Fischman AM, Nowakowski FS, Ellozy SH, Faries PL, Marin ML, Lookstein RA. Anatomic risk factors for type-2 endoleak following EVAR: a retrospective review of preoperative CT angiography in 326 patients. Cardiovasc Intervent Radiol. 2014;37(2):324–8.
Brown A, Saggu GK, Bown MJ, Sayers RD, Sidloff DA. Type II endoleaks: challenges and solutions. Vasc Health Risk Manag. 2016;2(12):53–63.
Walker J, Tucker LY, Goodney P, Candell L, Hua H, Okuhn S, Hill B, Chang RW. Type II endoleak with or without intervention after endovascular aortic aneurysm repair does not change aneurysm-related outcomes despite sac growth. J Vasc Surg. 2015;62(3):551–61.
Karthikesalingam A, Thrumurthy SG, Jackson D, Choke E, Sayers RD, Loftus IM, Thompson MM, Holt PJ. Current evidence is insufficient to define an optimal threshold for intervention in isolated type II endoleak after endovascular aneurysm repair. J Endovasc Ther. 2012;19(2):200–8.
Jones JE, Atkins MD, Brewster DC, Chung TK, Kwolek CJ, LaMuraglia GM, Hodgman TM, Cambria RP. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg. 2007;46(1):1–8.
van Marrewijk CJ, Fransen G, Laheij RJ, Harris PL, Buth J. EUROSTAR collaborators. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg. 2004;27(2):128–37.
Cieri E, De Rango P, Isernia G, Simonte G, Ciucci A, Parlani G, Verzini F, Cao P. Type II endoleak is an enigmatic and unpredictable marker of worse outcome after endovascular aneurysm repair. J Vasc Surg. 2014;59(4):930–7.
Aziz A, Menias CO, Sanchez LA, Picus D, Saad N, Rubin BG, Curci JA, Geraghty PJ. Outcomes of percutaneous endovascular intervention for type II endoleak with aneurysm expansion. J Vasc Surg. 2012;55(5):1263–7.
Hongo N, Kiyosue H, Shuto R, Kamei N, Miyamoto S, Tanoue S, Mori H. Double coaxial microcatheter technique for transarterial aneurysm sac embolisation of type II endoleaks after endovascular abdominal aortic repair. J Vasc Interv Radiol. 2014;25(5):709–16.
Haulon S, Tyazi A, Willoteaux S, Koussa M, Lions C, Beregi JP. Embolisation of type II endoleaks after aortic stent-graft implantation: technique and immediate results. J Vasc Surg. 2001;34(4):600–5.
Bosiers MJ, Schwindt A, Donas KP, Torsello G. Midterm results of the transarterial use of Onyx in the treatment of persisting type II endoleaks after EVAR. J Cardiovasc Surg Torino. 2013;54(4):469–75.
Gallagher KA, Ravin RA, Meltzer AJ, Khan MA, Coleman DM, Graham AR, Aiello F, Shrikhande G, Connolly PH, Dayal R, Karwowski JK. Midterm outcomes after treatment of type II endoleaks associated with aneurysm sac expansion. J Endovasc Ther. 2012;19(2):182–92.
Kasirajan K, Matteson B, Marek JM, Langsfeld M. Technique and results of transfemoral superselective coil embolisation of type II lumbar endoleak. J Vasc Surg. 2003;38(1):61–6.
Kajiwara K, Yamagami T, Urashima M, Tomiyoshi H, Kakizawa H, Yoshimatsu R, Ishikawa M, Awai K. Embolisation for type 2 endoleak with sac expansion after endovascular repair of abdominal aortic aneurysm: safety and effectiveness. Springerplus. 2016;2(5):262.
Müller-Wille R, Wohlgemuth WA, Heiss P, Wiggermann Ph, Güntner O, Schreyer AS, Hoffstetter P, Chr S, Zorger N. Transarterial embolisation of type II endoleaks after EVAR: the role of ethylene vinyl alcohol copolymer (Onyx). Cardiovasc Intervent Radiol. 2013;36(5):1288–95.
William Stavropoulos SW, Park J, Fairman R, Carpenter J. Type 2 Endoleak embolisation comparison: translumbar embolisation versus modified transarterial embolisation. J Vasc Interv Radiol. 2009;20:1299–302.
Arenas Azofra E, Rey VM, Marcos FÁ, Al-Sibbai AZ, García FV, Pérez MA. Results of transarterial embolisation for treating type 2 endoleaks: a single-center experience. Ann Vasc Surg. 2020;66:104–9.
Zener R, Oreopoulos G, Beecroft R, Rajan DK, Jaskolka J, Tan KT. Transabdominal direct sac puncture embolisation of type II endoleaks after endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol. 2018;29(8):1167–73.
Moosavi B, Kaitoukov Y, Khatchikian A, Bayne JP, Constantin A, and Camlioglu E. Direct sac puncture versus transarterial embolisation of type II endoleaks after endovascular abdominal aortic aneurysm repair: Comparison of outcomes.
Carrafiello G, Ierardi AM, Radaelli A, et al. Unenhanced cone beam computed tomography and fusion imaging in direct percutaneous sac injection for treatment of Type II endoleak: technical note. Cardiovasc Intervent Radiol. 2016;39:447–52.
Barleben A, Quinones-Baldrich W, Mogannam A, Archie M, Lane JS, Malas M. Midterm evaluation of perigraft arterial sac embolisation in endovascular aneurysm repair. J Vasc Surg. 2020;72(6):1960–7.
Ameli-Renani S, Pavlidis V, Mailli L, et al. Transiliac paraendograft embolisation of type 2 endoleak: an alternative approach for endoleak management. Cardiovasc Intervent Radiol. 2016;39:279–83.
Coppi G, Saitta G, Gennai S, et al. Transealing: a novel and simple technique for embolisation of type 2 endoleaks through direct sac access from the distal stent-graft landing zone. Eur J Vasc Endovasc Surg. 2014;47:394–401.
Giles KA, Fillinger MF, De Martino RR, et al. Results of transcaval embolisation for sac expansion from type II endoleaks after endovascular aneurysm repair. J Vasc Surg. 2015;61:1129–36.
Gandini R, Chiocchi M, Loreni G, et al. Treatment of type II endoleak after endovascular aneurysm repair: the role of selective versus nonselective transcaval embolisation. J Endovasc Ther. 2014;21:714–22.
Hyatt E, McLaughlin JN, Shah H, Kalva SP. Transcaval approach for embolisation of type II Endoleak following endovascular aortic aneurysm repair. CVIR Endovasc. 2019;2(1):3.
Van Sickler AP, Smith AH, Ellis RC, Steenberge SP, Quatromoni JG, Rowse JW, Smolock Chr J, Caputo FJ, Kirksey L. A novel technique and outcomes for transcaval endoleak embolisation. Ann Vasc Surg. 2023;93:300–7.
Maleux G, Poorteman L, Laenen A, Saint-Lèbes B, Houthoofd S, Fourneau I, Rousseau H. Incidence, etiology, and management of type III endoleak after endovascular aortic repair. J Vasc Surg. 2017;66(4):1056–64.
Forsyth A, Carlson S, Martin M, Raffetto J, Alfson D, McPhee J. Late type III endoleaks are common in early generation Endologix AFX stent grafts. J Vasc Surg. 2022;76(3):680–7.
Update on Endologix AFX Endovascular AAA Graft Systems and Risk of Type III Endoleak: FDA Safety Communication | FDA. 2022 Dec. Available at: https://www.fda.gov/medical-devices/safety-communications/update-endologix-afx-endovascular-aaa-graft-systems-and-risk-type-iii-endoleak-fda-safety
Blakeslee-Carter J, Beck AW, Spangler EL. Type III endoleaks in complex endovascular abdominal aortic aneurysm repair within the vascular quality initiative. J Vasc Surg. 2022;75(4):1172–80.
Deslarzes-Dubuis C, Stern JR, Tran K, Colvard B, Lee JT. Fenestrated endovascular repair with large device diameters (34–36 mm) is associated with type 1 and 3 endoleak and reintervention. Ann Vasc Surg. 2022;80:235–40.
Zoethout AC, Ketting S, Zeebregts CJ, Apostolou D, Mees BME, Berg P, Beyrouti HE, De Vries JPM, Torella F, Migliari M, Silingardi R, Reijnen MMPJ. An international, multicenter retrospective observational study to assess technical success and clinical outcomes of patients treated with an endovascular aneurysm sealing device for type III endoleak. J Endovasc Ther. 2022;29(1):57–65.
Stoecker JB, Glaser JD. Review of type III endoleaks. Semin Intervent Radiol. 2020;37(4):371–6.
Dossabhoy SS, Simons JP, Diamond KR, Flahive JM, Aiello FA, Arous EJ, Messina LM, Schanzer A. Reinterventions after fenestrated or branched endovascular aortic aneurysm repair. J Vasc Surg. 2018;68(3):669–81.
White SB, Stavropoulos SW. Management of endoleaks following endovascular aneurysm repair. Semin Intervent Radiol. 2009;26(1):33–8.
Schuurman JP, Fioole B, van den Heuvel DA, de Vries JP. Endovascular therapy for recurrent type III endoleak. Vasc Endovascular Surg. 2010;44(2):123–5.
van Sambeek MR, Hendriks JM, Tseng L, van Dijk LC, van Urk H. Sac enlargement without endoleak: when and how to convert and technical considerations. Semin Vasc Surg. 2004;17(4):284–7.
Torres-Blanco Á, Miralles-Hernández M. Endotension: twenty years of a controversial term. CVIR Endovasc. 2021;4(1):46.
Chen J, Stavropoulos S. Management of endoleaks. Semin Intervent Radiol. 2015;32(3):259–64.
Parsa P, Das Gupta J, McNally M, Chandra V. Endotension: What do we know and not know about this enigmatic complication of endovascular aneurysm repair. J Vasc Surg. 2021;74(2):639–45.
Patel S, Chun JY, Morgan R. Enlarging aneurysm sac post EVAR - type V or occult type II endoleak? CVIR Endovasc. 2023;6(1):4.
Torikai H, Inoue M, Nakatsuka S, Tamura M, Yashiro H, Yoshitake A, Shimizu H, Jinzaki M. Imaging findings of atypical type II endoleak through vasa vasorum after abdominal endovascular aneurysm repair. Cardiovasc Intervent Radiol. 2018;41(1):186–90.
Funaki B, Birouti N, Zangan M, et al. Evaluation and treatment of suspected type II endoleaks in patients with enlarging abdominal aortic aneurysms. J Vasc Interv Radiol. 2012;23(7):866–72.
Risberg B, Delle M, Eriksson E, et al. Aneurysm sac hygroma: a cause of endotension. J Endovasc Ther. 2001;8(5):447–53.
Risberg B, Delle M, Lönn L, et al. Management of aneurysm sac hygroma. J Endovasc Ther. 2004;11(2):191–5.
Ryu RK, Palestrant S, Ryu J, Trachtenberg J. Sac hygroma after endovascular abdominal aortic aneurysm repair: successful treatment with endograft relining. Cardiovasc Intervent Radiol. 2007;30(3):488–90.
Williams A, Williams Z. Imaging modalities for endoleak surveillance. J Med Radiat Sci. 2021;68(4):446–52.
Derboghossian T, Cavaye T, Quinn S, Pinto N. Symptomatic infrarenal aortic aneurysm sac expansion 5 years post-endovascular repair without an identifiable endoleak. Australas J Ultrasound Med. 2020;23(2):144–8.
Nakai M, Ikoma A, Loffroy R, Kamisako A, Higashino N, Sonomura T. Endovascular management of endotension by graft reinforcement followed by direct sac embolisation. Minim Invasive Ther Allied Technol. 2019;28(4):234–40.
Thoo C, Bourke B, May J. Symptomatic sac enlargement and rupture due to seroma after open abdominal aortic aneurysm repair with polytetrafluoroethylene graft: Implications for endovascular repair and endotension. J Vasc Surg. 2004;40(6):1089–94.
O’Donnell TFX, Corey MR, Deery SE, Tsougranis G, Maruthi R, Clouse WD, Cambria RP, Conrad MF. Select early type IA endoleaks after endovascular aneurysm repair will resolve without secondary intervention. J Vasc Surg. 2018;67(1):119–25.
Rajani RR, Arthurs ZM, Srivastava SD, Lyden SP, Clair DG, Eagleton MJ. Repairing immediate proximal endoleaks during abdominal aortic aneurysm repair. J Vasc Surg. 2011;53(5):1174–7.
Kim SM, Ra HD, Min SI, Jae HJ, Ha J, Min SK. Clinical significance of type I endoleak on completion angiography. Ann Surg Treat Res. 2014;86(2):95–9.
Perini P, Bianchini Massoni C, Mariani E, Ucci A, Fanelli M, Azzarone M, Freyrie A. Systematic review and meta-analysis of the outcome of different treatments for type 1a endoleak after EVAR. Ann Vasc Surg. 2019;60:435–46.
Patel S, Pavlidis V, Ameli-Renani S, Chun J-Y, Mailli L, Morgan R. Long-term outcomes following transarterial embolisation of proximal type I endoleaks post-EVAR. Cardiovasc Intervent Radiol. 2023;46:428–35.
Ierardi AM, Franchin M, Fontana F, Piffaretti G, Crippa M, Angileri SA, Magenta Biasina A, Piacentino F, Tozzi M, Pinto A, Carrafiello G. The role of ethylene-vinyl alcohol copolymer in association with other embolic agents for the percutaneous and endovascular treatment of type Ia endoleak. Radiol Med. 2018;123(8):638–42.
Marcelin C, Le Bras Y, Petitpierre F, Midy D, Grenier N, Ducasse E, Cornélis F. Embolisation for persistent type IA endoleaks after chimney endovascular aneurysm repair with Onyx®. Diagn Interv Imaging. 2017;98(12):849–55.
Graif A, Vance AZ, Garcia MJ, Lie KT, McGarry MK, Leung DA. Transcatheter embolisation of type I endoleaks associated with endovascular abdominal aortic aneurysm repair using ethylene vinyl alcohol copolymer. Vasc Endovasc Surg. 2017;51(1):28–32.
Eberhardt KM, Sadeghi-Azandaryani M, Worlicek S, Koeppel T, Reiser MF, Treitl M. Treatment of type I endoleaks using transcatheter embolisation with onyx. J Endovasc Ther. 2014;21(1):162–71.
Henrikson O, Roos H, Falkenberg M. Ethylene vinyl alcohol copolymer (Onyx) to seal type 1 endoleak. New Tech Vasc. 2011;19(2):77–81.
Lu Q, Feng J, Yang Y, Nie B, Bao J, Zhao Z, Feng X, Pei Y, Yuan L, Mei Z, Feng R, Jing Z. Treatment of type I endoleak after endovascular repair of infrarenal abdominal aortic aneurysm: success of fibrin glue sac embolisation. J Endovasc Ther. 2010;17(6):687–93.
Golzarian J, Maes EB, Sun S. Endoleak: treatment options. Tech Vasc Interv Radiol. 2005;8(1):41–9.
Maldonado TS, Rosen RJ, Rockman CB, Adelman MA, Bajakian D, Jacobowitz GR, Riles TS, Lamparello PJ. Initial successful management of type I endoleak after endovascular aortic aneurysm repair with n-butyl cyanoacrylate adhesive. J Vasc Surg. 2003;38(4):664–70.
Ameli-Renani S, Pavlidis V, Morgan RA. Early and midterm outcomes after transcatheter embolisation of type I endoleaks in 25 patients. J Vasc Surg. 2017;65(2):346–55.
Bianchini Massoni C, Perini P, Tecchio T, Azzarone M, de Troia A, Freyrie A. A systematic review of treatment modalities and outcomes of type 1b endoleak after endovascular abdominal aneurysm repair. Vascular. 2018;26:90–8.
Sarac TP, Gibbons C, Vargas L, Liu J, Srivastava S, Bena J, Mastracci T, Kashyap VS, Clair D. Long-term follow-up of type II endoleak embolisation reveals the need for close surveillance. J Vasc Surg. 2012;55(1):33–40.
Abularrage CJ, Patel VI, Conrad MF, Schneider EB, Cambria RP, Kwolek CJ. Improved results using Onyx glue for the treatment of persistent type 2 endoleak after endovascular aneurysm repair. J Vasc Surg. 2012;56(3):630–6.
Sheehan MK, Barbato J, Compton CN, Zajko A, Rhee R, Makaroun MS. Effectiveness of coiling in the treatment of endoleaks after endovascular repair. J Vasc Surg. 2004;40(3):430–4.
Horinouchi H, Okada T, Yamaguchi M, Maruyama K, Sasaki K, Gentsu T, Ueshima E, Sofue K, Kawasaki R, Nomura Y, Omura A, Okada K, Sugimoto K, Murakami T. Mid-term outcomes and predictors of transarterial embolisation for type ii endoleak after endovascular abdominal aortic aneurysm repair. Cardiovasc Intervent Radiol. 2020;43(5):696–705.
Wu WW, Swerdlow NJ, Dansey K, Shuja F, Wyers MC, Schermerhorn ML. Surgical treatment patterns and clinical outcomes of patients treated for expanding aneurysm sacs with type II endoleaks after endovascular aneurysm repair. J Vasc Surg. 2021;73(2):484–93.
Jouhannet C, Alsac JM, Julia P, Sapoval M, El Batti S, Di Primio M, Fabiani JN. Reinterventions for type 2 endoleaks with enlargement of the aneurismal sac after endovascular treatment of abdominal aortic aneurysms. Ann Vasc Surg. 2014;28(1):192–200.
Chun J, Morgan R. Ischaemic sequelae following glue embolisation of type 2 endoleak involving multiple lumbar arteries. Cardiovasc Intervent Radiol. 2020;43(9):1406–8.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
All authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chun, JY., de Haan, M., Maleux, G. et al. CIRSE Standards of Practice on Management of Endoleaks Following Endovascular Aneurysm Repair. Cardiovasc Intervent Radiol 47, 161–176 (2024). https://doi.org/10.1007/s00270-023-03629-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-023-03629-1