Abstract
Purpose
To explore the temperature threshold of thermal damage to growth plates.
Methods
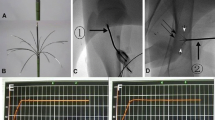
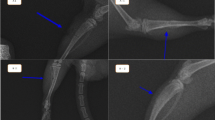
Nine rabbits were divided into three groups for femoral ablation, exposing the growth plate to different temperatures (T1 = 43–45 °C; T2 = 46–48 °C; T3 = 49–51 °C). After 5 weeks, the changes in the femurs were assessed by macroscopic images, micro-CT, haematoxylin and eosin staining, and immunohistochemistry of Col2a1 (type II collagen). At the cellular level, rabbit epiphyseal chondrocytes were exposed to 37 °C, 44 °C, 47 °C and 50 °C for 5 min. Then, proliferation and chondrogenic differentiation were detected.
Results
The rabbits in the T2 and T3 groups developed length discrepancies and axial deviations of femurs, abnormal newly formed bone in the marrow cavity, disorganized growth plates and decreased Col2a1 expression. At the cellular level, the cells exposed to 47 °C and 50 °C for 5 min showed decreased viability, increased apoptosis, decreased extracellular matrix synthesis and decreased matrix mineralization. However, the changes in rabbits in the T1 group and cells at 44 °C did not show a significant difference.
Conclusion
The ablation of growth plates at temperatures above 45 °C for 5 min results in decreased chondrocyte viability and disorganized growth plates, leading to growth disturbances. Further studies are warranted to confirm these promising initial results.




Similar content being viewed by others
References
Rinzler ES, et al. Microwave ablation of osteoid osteoma: initial experience and efficacy. Pediatr Radiol. 2019;49(4):566–70.
Fan QY, et al. Microwave ablation of primary malignant pelvic bone tumors. Front Surg. 2019;6:5.
Qiu YY, et al. Combination of microwave ablation and percutaneous osteoplasty for treatment of painful extraspinal bone metastasis. J Vasc Interv Radiol. 2019;30(12):1934–40.
Kastler A, et al. Analgesic effects of microwave ablation of bone and soft tissue tumors under local anesthesia. Pain Med. 2013;14(12):1873–81.
Good CR, et al. Glenohumeral chondrolysis after shoulder arthroscopy with thermal capsulorrhaphy. Arthroscopy. 2007;23(7):797e1–5.
van Eck CF, van Meel TAC, van den Bekerom MPJ, Zijl JAC, Bauke K. Heat-related complications from radiofrequency and electrocautery devices used in arthroscopic surgery: a systematic review. Arthrosc Sports Med Rehabil. 2021;3(2):e605–13.
Joerg Jerosch AMA. Chondrolysis of the glenohumeral joint following arthroscopic capsular release for adhesive capsulitis: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(3):292–4.
Friedman MV, et al. Hip chondrolysis and femoral head osteonecrosis: a complication of periacetabular cryoablation. J Vasc Interv Radiol. 2014;25(10):1580–8.
Christopher J, Hogan DRD. Progressive articular cartilage loss following radiofrequency treatment of a partial-thickness lesion. Arthroscopy. 2001;17(6):E24.
Orth P, Kohn D. Diagnostics and treatment of osteoid osteoma. Orthopade. 2017;46(6):510–21.
Jundt G, Baumhoer D. Chondroblastoma. Pathologe. 2018;39(2):132–8.
Wang C, et al. Osteochondroma of the talar neck: a case report and literature review. J Foot Ankle Surg. 2016;55(2):338–44.
McLeod RA, Dahlin DC, Beabout JW. The spectrum of osteoblastoma. AJR Am J Roentgenol. 1976;126(2):321–5.
van der Eerden BCJ, et al. Androgen receptor in the growth plate of the rat: Expression during male and female development and its regulation by gonadectomy. J Bone Miner Res. 2000;15:S326–S326.
Ierardi AM, et al. A new system of microwave ablation at 2450 MHz: preliminary experience. Updat Surg. 2015;67(1):39–45.
Springer B, et al. The influence of femoral and tibial bony anatomy on valgus OA of the knee. Knee Surg Sports Traumatol Arthrosc. 2020;28(9):2998–3006.
Kraus T, et al. The influence of biodegradable magnesium implants on the growth plate. Acta Biomater. 2018;66:109–17.
Du ZS, et al. Feasibility of microwave ablation of the vertebral growth plate for spine growth regulation: a preliminary study. Int J Hyperth. 2021;38(1):1233–41.
Shiguetomi-Medina JM, Moller-Madsen B, Rahbek O. Physeal histological morphology after thermal epiphysiodesis using radiofrequency ablation. J Orthop Traumatol. 2017;18(2):121–6.
Widmann RF, et al. Percutaneous radiofrequency epiphysiodesis in a rabbit model: a pilot study. Clin Orthop Relat Res. 2010;468(7):1943–8.
Voss JR, et al. Effects of thermal energy on chondrocyte viability. Am J Vet Res. 2006;67(10):1708–12.
Kaplan LD, et al. Recovery of chondrocyte metabolic activity after thermal exposure. Am J Sports Med. 2003;31(3):392–8.
Horstman CL, McLaughlin RM. The use of radiofrequency energy during arthroscopic surgery and its effects on intraarticular tissues. Vet Comp Orthop Traumatol. 2006;19(2):65–71.
Bauones S, et al. Protection of the proximal articular cartilage during percutaneous thermal ablation of acetabular metastasis using temperature monitoring. Cardiovasc Intervent Radiol. 2018;41(1):163–9.
Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
Michigami T. Regulatory mechanisms for the development of growth plate cartilage. Cell Mol Life Sci. 2013;70(22):4213–21.
Hendrickx G, et al. Piezo1 inactivation in chondrocytes impairs trabecular bone formation. J Bone Miner Res. 2021;36(2):369–84.
Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35(5):1003–12.
Nakahara S, et al. A porcine study of the area of heated tissue during hot-balloon ablation: Implications for the clinical efficacy and safety. J Cardiovasc Electrophysiol. 2021;32(2):260–9.
Korganbayev S, et al. Closed-loop temperature control based on fiber bragg grating sensors for laser ablation of hepatic tissue. Sensors. 2020;20(22):6496.
Gangi A, et al. “Keeping a cool head”: percutaneous imaging-guided cryo-ablation as salvage therapy for recurrent glioblastoma and head and neck tumours. Cardiovasc Intervent Radiol. 2020;43(2):172–5.
Tsoumakidou G, et al. Image-guided spinal ablation: a review. Cardiovasc Intervent Radiol. 2016;39(9):1229–38.
Chu CR, et al. Recovery of articular cartilage metabolism following thermal stress is facilitated by IGF-1 and JNK inhibitor. Am J Sports Med. 2004;32(1):191–6.
Kurup AN, et al. Avoiding complications in bone and soft tissue ablation. Cardiovasc Intervent Radiol. 2017;40(2):166–76.
Hajdu S, et al. The effect of drilling and screw fixation of the growth plate–an experimental study in rabbits. J Orthop Res. 2011;29(12):1834–9.
Funding
The work was supported by Major Program of Hubei Technical Innovation Special Project (Grant number 2017ACA098).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Consent for Publication
For this type of study, consent for publication is not required.
Ethical Approval
All applicable institutional guidelines for the care and use of animals were followed.
Informed Consent
For this type of study, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Yan, F., Zhong, A. et al. Effect of Thermal Ablation on Growth Plates: A Study to Explore the Thermal Threshold of Rabbit Growth Plates During Microwave Ablation. Cardiovasc Intervent Radiol 46, 112–119 (2023). https://doi.org/10.1007/s00270-022-03238-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03238-4