Abstract
Purpose
To evaluate the safety and efficacy of bleomycin infusion sclerotherapy using a syringe pump in microcystic and mixed (microcystic components with the presence of a cyst over 1 cm) lymphatic malformations (LMs).
Materials and Methods
Patients who received bleomycin sclerotherapy with a syringe pump for microcystic or mixed LMs were reviewed. Cystic components of LMs were accessed under sonographic guidance, followed by injection of an opacified bleomycin solution using a syringe pump (infusion rate, 10–20 mL/h) under fluoroscopic guidance. Imaging outcomes were graded as complete (> 90% size reduction), partial (25–90%), or no response (< 25%). Clinical outcomes and procedure-related complications were also reviewed.
Results
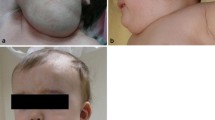
Forty-nine patients with 81 sclerotherapies were analyzed. The mean age was 17 years (range 0.1–65 y). Thirty-one (63%) patients had microcystic LMs, and 18 (37%) had mixed. A mean of 1.7 sessions (range 1–4) of sclerotherapy was performed using a mean cumulative dose of bleomycin of 10.8 U (range 1.5–39 U). The mean infusion time was 39 min (range 14–130 min). Regarding imaging outcomes, there was a complete response in 29% (n = 14), a partial response in 57% (n = 28), and no response in 14% (n = 7). Regarding clinical outcomes, there was a complete response in 39% (n = 19), a partial response in 51% (n = 25), and no response in 10% (n = 5). According to the CIRSE classification, no major complications were identified.
Conclusions
Bleomycin slow infusion sclerotherapy provides gradual filling of sclerosant to target microcystic components. This technique is safe and feasible for the management of microcystic or mixed LMs.
Level of Evidence
Level 4, Case series.



Similar content being viewed by others
Abbreviations
- LM:
-
Lymphatic malformation
- MRI:
-
Magnetic resonance imaging
References
The International Society for the Study of Vascular Anomalies (ISSVA). ISSVA classification. 2018. Available online: https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf. Accessed 2 Feb 2022.
Giguere CM, Bauman NM, Sato Y, Burke DK, Greinwald JH, Pransky S, et al. Treatment of lymphangiomas with OK-432 (Picibanil) sclerotherapy: a prospective multi-institutional trial. Arch Otolaryngol Head Neck Surg. 2002;128(10):1137–44.
Chaudry G, Burrows PE, Padua HM, Dillon BJ, Fishman SJ, Alomari AI. Sclerotherapy of abdominal lymphatic malformations with doxycycline. J Vasc Interv Radiol. 2011;22(10):1431–5.
Shergill A, John P, Amaral JG. Doxycycline sclerotherapy in children with lymphatic malformations: outcomes, complications and clinical efficacy. Pediatr Radiol. 2012;42(9):1080–8.
Sainsbury DCG, Kessell G, Fall AJ, Hampton FJ, Guhan A, Muir T. Intralesional bleomycin injection treatment for vascular birthmarks: a 5-year experience at a single United Kingdom unit. Plast Reconstr Surg. 2011;127(5):2031–44.
Muir T, Kirsten M, Fourie P, Dippenaar N, Ionescu GO. Intralesional bleomycin injection (IBI) treatment for haemangiomas and congenital vascular malformations. Pediatr Surg Int. 2004;19(12):766–73.
Jin Y, Zou Y, Hua C, Chen H, Yang X, Ma G, et al. Treatment of early-stage extracranial arteriovenous malformations with intralesional interstitial bleomycin injection: a pilot study. Radiology. 2018;287(1):194–204.
Chaudry G, Guevara CJ, Rialon KL, Kerr C, Mulliken JB, Greene AK, et al. Safety and efficacy of bleomycin sclerotherapy for microcystic lymphatic malformation. Cardiovasc Intervent Radiol. 2014;37(6):1476–81.
Lee J, Lee SJ, Chung HY, et al. Infusion sclerotherapy of microcystic lymphatic malformation: clinico-radiological mid-term results. J Korean Soc Radiol. 2016;74:26–36.
Da Ros V, Iacobucci M, Puccinelli F, Spelle L, Saliou G. Lymphographic-like technique for the treatment of microcystic lymphatic malformation components of < 3 mm. AJNR Am J Neuroradiol. 2018;39(2):350–4.
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40(8):1141–6.
Umezawa H, Ishizuka M, Maeda K, Takeuchi T. Studies on bleomycin. Cancer. 1967;20(5):891–5.
Lopez-Larraza D, De Luca JC, Bianchi NO. The kinetics of DNA damage by bleomycin in mammalian cells. Mutat Res. 1990;232(1):57–61.
Blum RH, Carter SK, Agre K. A clinical review of bleomycin—a new antineoplastic agent. Cancer. 1973;31(4):903–14.
Ohta H, Chiba S, Ebina M, Furuse M, Nukiwa T. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302(2):L193-205.
McMorrow L, Shaikh M, Kessell G, Muir T. Bleomycin electrosclerotherapy: new treatment to manage vascular malformations. Br J Oral Maxillofac Surg. 2017;55(9):977–9.
Wohlgemuth WA, Muller-Wille R, Meyer L, Wildgruber M, Guntau M, Heydt SV, et al. Bleomycin electrosclerotherapy in therapy-resistant venous malformations of the body. J Vasc Surg Venous Lymphat Disord. 2021;9(3):731–9.
Zhang W, Chen G, Ren JG, Zhao YF. Bleomycin induces endothelial mesenchymal transition through activation of mTOR pathway: a possible mechanism contributing to the sclerotherapy of venous malformations. Br J Pharmacol. 2013;170(6):1210–20.
Levy RL, Chiarillo S. Hyperpyrexia, allergic-type response and death occurring with low-dose bleomycin administration. Oncology. 1980;37(5):316–7.
Mendez-Echevarria A, Fernandez-Prieto A, de la Serna O, Lopez-Gutierrez JC, Parron M, Marin-Aguilera B, et al. Acute lung toxicity after intralesional bleomycin sclerotherapy. Pediatrics. 2018;141(1).
O’Sullivan JM, Huddart RA, Norman AR, Nicholls J, Dearnaley DP, Horwich A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol. 2003;14(1):91–6.
Comis RL. Bleomycin pulmonary toxicity: current status and future directions. Semin Oncol. 1992;19(2 Suppl 5):64–70.
Acknowledgements
We would like to thank Wade Martin of Emareye Medical Editing for his critical revision of this manuscript.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. Institutional Review Board (IRB) approval was obtained for this study.
Informed Consent
Indivisual written informed consents for the sclerotherapy procedures were obtained from all patients or their authorized trustees.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cha, J.G., Lee, J., Lee, S.Y. et al. Safety and Efficacy of Bleomycin Slow Infusion Sclerotherapy Using a Syringe Pump for Microcystic and Mixed Lymphatic Malformations. Cardiovasc Intervent Radiol 45, 1288–1294 (2022). https://doi.org/10.1007/s00270-022-03224-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03224-w