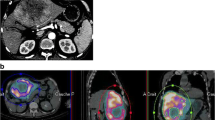
Abstract
Trans-arterial radioembolization is currently performed using 90Y-loaded glass or resin microspheres and also using 166Ho-loaded microspheres. The goal of this review is to present dosimetry and radiobiology concepts, the different dosimetry approaches available (simulation-based dosimetry and post-treatment dosimetry), main confounding factors as main clinical dosimetry results provided during the last decade for both hepatocellular carcinoma (HCC) and metastases of colorectal carcinoma (mCRC). Based on the different number of microspheres or different isotope used, radiobiology of the three devices is different, meaning that tumouricidal doses and maximal tolerated doses are different. Tumouricidal doses described for HCCs were 100–120 grays (Gy) with 90Y resin microspheres and 205 Gy with 90Y glass microspheres. For mCRC, it is 39–60 with 90Y resin microspheres, 139 Gy with 90Y glass microspheres and 90 Gy with 166Ho microspheres. An impact of tumoural doses with overall survival has also been reported. Personalised dosimetry has been developed and is now recommended by several international expert groups. Level-one evidence of the major impact of personalised dosimetry on response and overall survival in HCC is now available, bringing a new standard approach for TARE in clinical practice as well as for trial design.

Similar content being viewed by others
References
Garin E, Rolland Y, Laffont S, Edeline J. Clinical impact of (99m)Tc-MAA SPECT/CT-based dosimetry in the radioembolization of liver malignancies with (90)Y-loaded microspheres. Eur J Nucl Med Mol Imag. 2016;43:559–75.
Braat AJAT, Prince JF, van Rooij R, Bruijnen RCG, van den Bosch MAAJ, Lam MGEH. Safety analysis of holmium-166 microsphere scout dose imaging during radioembolisation work-up: a cohort study. Eur Radiol. 2018;28(3):920–8.
SIR-Spheres® Y-90 resin microspheres [package insert]. St Leonards, New South Wales, Australia: Sirtex Medical Pty Ltd; December 2019. https://www.sirtex.com/au/clinicians/package-insert/
TheraSphere™ Yttrium-90 Glass Microspheres [package insert]. Marlborough, MA; Boston Scientific Corporation; 1999. https://btgplc.com/BTG/media/TheraSphere-Documents/PDF/10093509-Rev8_English-searchable.pdf
Walrand S, Hesse M, Chiesa C, Lhommel R, Jamar F. The low hepatic toxicity per Gray of 90Y glass microspheres is linked to their transport in the arterial tree favoring a nonuniform trapping as observed in posttherapy PET imaging. J Nucl Med. 2014;55(1):135–40.
Pasciak AS, Abiola G, Liddell RP, Crookston N, Besharati S, Donahue D, et al. The number of microspheres in Y90 radioembolization directly affects normal tissue radiation exposure. Eur J Nucl Med Mol Imag. 2020;47(4):816–27. https://doi.org/10.1007/s00259-019-04588-x
Chiesa C, Maccauro M, Romito R, Spreafico C, Pellizzari S, Negri A, et al. Need, feasibility and convenience of dosimetric treatment planning in liver selective internal radiation therapy with (90)Y microspheres: the experience of the National Tumour Institute of Milan. Quart J Nucl Med Mol Imag. 2011;55:168–97.
Garin E, Lenoir L, Rolland Y, Laffont S, Pracht M, Mesbah H, et al. Effectiveness of quantitative MAA SPECT/CT for the definition of vascularized hepatic volume and dosimetric approach: phantom validation and clinical preliminary results in patients with complex hepatic vascularization treated with yttrium-90-labeled microspheres. Nucl Med Commun. 2011;32(12):21245–55.
Garin E, Rolland Y, Edeline J. 90Y-loaded microsphere SIRT of HCC patients with portal vein thrombosis: high clinical impact of 99mTc-MAA SPECT/CT-based dosimetry. Semin Nucl Med. 2019;49(3):218–26.
Garin E, Palard X, Rolland Y. Personalised dosimetry in radioembolisation for HCC: impact on clinical outcome and on trial design. Cancers (Basel). 2020;12(6).
Kafrouni M, Allimant C, Fourcade M, Vauclin S, Guiu B, Mariano-Goulart D, et al. Analysis of differences between (99m)Tc-MAA SPECT- and (90)Y-microsphere PET-based dosimetry for hepatocellular carcinoma selective internal radiation therapy. EJNMMI Res. 2019;9(1):62.
Haste P, Tann M, Persohn S, La Roche T, Aaron V, Mauxion T, et al. Correlation of Technetium-99m Macroaggregated Albumin and Yttrium-90 Glass microsphere biodistribution in hepatocellular Carcinoma: a retrospective review of pretreatment single photon emission CT and posttreatment positron emission tomography/CT. J Vasc Interv Radiol. 2017;28:722–30.
Wondergem M, Smits ML, Elschot M, de Jong HW, Verkooijen HM, van den Bosch MA, et al. 99mTc-macroaggregated albumin poorly predicts the intrahepatic distribution of 90Y resin microspheres in hepatic radioembolization. J Nucl Med. 2013;54:1294–301.
Salem R, Padia SA, Lam M, Bell J, Chiesa C, Fowers K, et al. Clinical and dosimetric considerations for Y90: recommendations from an international multidisciplinary working group. Eur J Nucl Med Mol Imag. 2019;46(8):1695–704.
Garin E, Laurence Lenoir L, Yan Rolland Y, Julien Edeline J, Habiba Mesba H, et al. 99mTc-MAA SPECT/CT based dosimetry accurately predicts tumour response and survival in HCC patients treated with 90Y-loaded glass microspheres: preliminary results. J Nucl Med. 2012;53(2):255–63.
Van de Wiele C, Maes A, Brugman E, D’Asseler Y, De Spiegeleer B, Mees G, et al. SIRT of liver metastases: physiological and pathophysiological considerations. Eur J Nucl Med Mol Imag. 2012;39(10):1646–55.
Flamen P, Vanderlinden B, Delatte P, Ghanem G, Ameye L, Van Den Eynde M, et al. Multimodality imaging can predict the metabolic response of unresectable colorectal liver metastases to radioembolization therapy with Yttrium-90 labeled resin microspheres. Phys Med Biol. 2008;53(22):6591–603.
d’Abadie P, Walrand S, Hesse M, Annet L, Borbath I, Van den Eynde M, et al. Prediction of tumour response and patient outcome after radioembolization of hepatocellular carcinoma using 90Y-PET-computed tomography dosimetry. Nucl Med Commun. 2021;42(7):747–54.
Martin M, Hocquelet A, Debordeaux F, Bordenave L, Blanc JF, Papadopoulos P, et al. Comparison of perfused volume segmentation between cone-beam CT and (99m)Tc-MAA SPECT/CT for treatment dosimetry before selective internal radiation therapy using (90)Y-glass microspheres. Diagn Interv Imag. 2021;102(1):45–52.
Sueyoshi E, Hayashida T, Sakamoto I, Uetani M. Vascular complications of hepatic artery after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2010;195(1):245–51. https://doi.org/10.2214/AJR.08.2301.
Elice F, Francesco RF. Side effects of anti-angiogenic drugs. Thromb Res. 2012;129(Suppl 1):S50–3. https://doi.org/10.1016/S0049-3848(12)70016-6.
Elschot M, Nijsen JF, Lam MG, Smits ML, Prince JF, Viergever MA, et al. (99m)Tc-MAA overestimates the absorbed dose to the lungs in radioembolization: a quantitative evaluation in patients treated with (166)Ho-microspheres. Eur J Nucl Med Mol Imag. 2014;41(10):1965–75.
Ulrich G, Dudeck O, Furth C, Ruf J, Grosser OS, Adolf D, et al. Predictive value of intratumourtumoural 99mTc-macroaggregated albumin uptake in patients with colorectal liver metastases scheduled for radioembolization with 90Y-microspheres. J Nucl Med. 2013;54(4):516–22.
Ilhan H, Goritschan A, Paprottka P, Jakobs TF, Fendler WP, Todica A, et al. Predictive Value of 99mTc-MAA SPECT for 90Y-Labeled Resin Microsphere Distribution in Radioembolization of Primary and Secondary Hepatic Tumours. J Nucl Med. 2015;56(11):1654–60.
Kucuk ON, Soydal C, Araz M, Ozkan E, Aras G. Evaluation of the response to selective internal radiation therapy in patients with hepatocellular cancer according to pretreatment (99m)Tc-MAA uptake. Clin Nucl Med. 2013;38(4):252–5.
Willowson KP, Hayes AR, Chan DLH, Tapner M, Bernard EJ, Maher R, et al. Clinical and imaging-based prognostic factors in radioembolisation of liver metastases from colorectal cancer: a retrospective exploratory analysis. EJNMMI Res. 2017;7(1):46.
Gnesin S, Canetti L, Adib S, Cherbuin N, Silva Monteiro M, Bize P, et al. Partition model-based 99mTc-MAA SPECT/CT predictive dosimetry compared with 90Y TOF PET/CT posttreatment dosimetry in radioembolization of hepatocellular carcinoma: a quantitative agreement comparison. J Nucl Med. 2016;57(11):1672–8.
Jadoul A, Bernard C, Lovinfosse P, Gérard L, Lilet H, Cornet O, et al. Comparative dosimetry between 99mTc-MAA SPECT/CT and 90Y PET/CT in primary and metastatic liver tumours. Eur J Nucl Med Mol Imag. 2020;47(4):828–37.
Richetta E, Pasquino M, Poli M, Cutaia C, Valero C, Tabone M, et al. PET-CT post therapy dosimetry in radioembolization with resin 90Y microspheres: comparison with pre-treatment SPECT-CT 99mTc-MAA results. Phys Med. 2019;64:16–23.
Rhee S, Kim S, Cho J, Park J, Eo JS, Park S, et al. Semi-Quantitative Analysis of Post-Transarterial Radioembolization (90)Y microsphere Positron Emission Tomography Combined with Computed Tomography (PET/CT) images in advanced liver malignancy: comparison with (99m)Tc Macroaggregated Albumin (MAA) Single Photon Emission Computed Tomography (SPECT). Nucl Med Mol Imag. 2016;50(1):63–9.
d’Abadie P, Walrand S, Hesse M, Amini N, Lhommel R, Sawadogo K, et al. Accurate non-tumourtumoural 99mTc-MAA absorbed dose prediction to plan optimized activities in liver radioembolization using resin microspheres. Phys Med. 2021;89:250–7.
Jafargholi Rangraz E, Tang X, Van Laeken C, Maleux G, Dekervel J, Van Cutsem E, et al. Quantitative comparison of pre-treatment predictive and post-treatment measured dosimetry for selective internal radiation therapy using cone-beam CT for tumour and liver perfusion territory definition. EJNMMI Res. 2020;10(1):94.
Skanjeti A, Magand N, Defez D, Tordo J, Rode A, Manichon AF. Selective internal radiation therapy of hepatic tumours: morphologic and functional imaging for voxel-based computer-aided dosimetry. Biomed Pharmacother. 2020;132: 110865.
Garin E, Lenoir L, Edeline J, Laffont S, Mesbah H, Poree P, et al. Boosted selective internal radiation therapy with 90Y-loaded glassmicrospheres (B-SIRT) for hepatocellular carcinoma patients: anew personalized promising concept. Eur J Nucl Med MolImag. 2013;40:1057–68.
Garin E, Rolland Y, Edeline J, Icard N, Lenoir L, Laffont S, et al. Personalized dosimetry and intensification concept with 90Y-loaded glass microsphere radioembolization induce prolonged overall survival in hepatocelluar carcinoma patients with portal vein thrombosis. J Nucl Med. 2015;56(3):339–46.
Garin E, Rolland Y, Pracht M, Le Sourd S, Laffont S, Mesbah H, et al. High impact of macroaggregated albumin-based tumour dose on response and overall survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microsphere radioembolization. Liver Int. 2017;37(1):101–10.
Ho CL, Chen S, Cheung SK, Leung YL, Cheng KC, Wong KN, et al. Radioembolization with 90Y glass microspheres for hepatocellular carcinoma: significance of pre-treatment 11C-acetate and 18F-FDG PET/CT and post-treatment 90Y PET/CT in individualized dose prescription. Eur J Nucl Med Mol Imag. 2018;45:2110–21.
Lau WY, Leung WT, Ho S, Leung NW, Chan M, Lin J, et al. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer. 1994;70(5):994–9.
Hermann AL, Dieudonné A, Ronot M, Sanchez M, Pereira H, Chatellier G, et al. SARAH Trial Group. Relationship of tumour radiation-absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with 90Y in the SARAH study. Radiology. 2020;296(3):673–84.
Kao YH, Hock Tan AE, Burgmans MC, Irani FG, Khoo LS, Gong Lo RH, et al. Image-guided personalized predictive dosimetry by artery-specific SPECT/CT partition modeling for safe and effective 90Y radioembolization. J Nucl Med. 2012;53(4):559–66.
Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–36.
Allimant C, Kafrouni M, Delicque J, Ilonca D, Cassinotto C, Assenat E, et al. Tumour targeting and three-dimensional voxel-based dosimetry to predict tumour response, toxicity, and survival after Yttrium-90 resin microsphere radioembolization in hepatocellular carcinoma. J Vasc Interv Radiol. 2018;29:1662–70.
Strigari L, Sciuto R, Rea S, Carpanese L, Pizzi G, Soriani A, et al. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: radiobiologic considerations. J Nucl Med. 2010;51:1377–85.
Chan KT, Alessio AM, Johnson GE, Vaidya S, Kwan SW, Monsky W, et al. Prospective trial using internal pair-production positron emission tomography to establish the Yttrium-90 radioembolization dose required for response of hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2018;101:358–65.
Kappadath SC, Mikell J, Balagopal A, Baladandayuthapani V, Kaseb A, Mahvash A. Hepatocellular carcinoma tumour dose response after (90)Y-radioembolization with glass microspheres using (90)Y-SPECT/CT-based voxel dosimetry. Int J Radiat Oncol Biol Phys. 2018;102:451–61.
Levillain H, Duran Derijckere I, Marin G, Guiot T, Vouche M, Reynaert N, et al. (90)Y-PET/CT-based dosimetry after selective internal radiation therapy predicts outcome in patients with liver metastases from colorectal cancer. EJNMMI Res. 2018;8(1):60.
Van den Hoven AF, Rosenbaum CE, Elias SG, de Jong HW, Koopman M, Verkooijen HM, et al. Insights into the dose response relationship of radioembolization with resin 90Y-microspheres: a prospective cohort study in patients with colorectal cancer liver metastases. J Nucl Med. 2016;57(7):1014–9.
Alsultan AA, van Roekel C, Barentsz MW, Smits MLJ, Kunnen B, Koopman M, et al. Dose-response and dose-toxicity relationships for yttrium-90 glass radioembolization in patients with colorectal cancer liver metastases. J Nucl Med. 2021. https://doi.org/10.2967/jnumed.120.255745.
van Roekel C, Bastiaannet R, Smits MLJ, Bruijnen RC, Braat AJAT, de Jong HWAM, et al. Dose-effect relationships of 166Ho Radioembolization in colorectal cancer. J Nucl Med. 2021;62(2):272–9.
Bastiaannet R, van Roekel C, Smits MLJ, Elias SG, van Amsterdam WAC, Doan D, et al. First evidence for a dose-response relationship in patients treated with 166Ho Radioembolization: a prospective study. J Nucl Med. 2020;61(4):608–12.
Chiesa C, Mira M, Maccauro M, Spreafico C, Romito R, Morosi C, et al. Radioembolization of hepatocarcinoma with (90)Y glass microspheres: development of an individualized treatment planning strategy based on dosimetry and radiobiology. Eur J Nucl Med Mol Imag. 2015;42:1718–38.
Sangro B, Gil-Alzugaray B, Rodriguez J, Sola I, Martinez-Cuesta A, Viudez A, et al. Liver disease induced by radioembolization of liver tumour; description and possible risk factors. Cancer. 2008;112(7):1538–46.
Chiesa C, Mira M, Bhoori S, Bormolini G, Maccauro M, Spreafico C, et al. Radioembolization of hepatocarcinoma with 90Y glass microspheres: treatment optimization using the dose-toxicity relationship. Eur J Nucl Med Mol Imag. 2020;47(13):3018–32.
Chan KT, Alessio AM, Johnson GE, Vaidya S, Kwan SW, Monsky W, et al. Hepatotoxic dose thresholds by positron-emission tomography after Yttrium-90 radioembolization of liver tumours: a prospective single-arm observational study. Cardiovasc Intervent Radiol. 2018;41(9):1363–72.
Smits ML, Nijsen JF, van den Bosch MA, Lam MG, Vente MA, Mali WP, et al. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol. 2012;13(10):1025–34.
Braat MNGJA, de Jong HW, Seinstra BA, Scholten MV, van den Bosch MAAJ, Lam MGEH. Hepatobiliary scintigraphy may improve radioembolization treatment planning in HCC patients. EJNMMI Res. 2017;7(1):2. https://doi.org/10.1186/s13550-016-0248-x.
Allimant C, Deshayes E, Kafrouni M, Santoro L, de Verbizier D, Fourcade M, et al. Hepatobiliary Scintigraphy and Glass 90Y Radioembolization with Personalized Dosimetry: Dynamic Changes in Treated and Nontreated Liver. Diagnostics (Basel). 2021;11(6):931. https://doi.org/10.3390/diagnostics11060931 (PMID: 34064296).
Palard X, Edeline J, Rolland Y, Le Sourd S, Pracht M, Laffont S, et al. Dosimetric parameters predicting contralateral liver hypertrophy after unilobar radioembolization of hepatocellular carcinoma. Eur J Nucl Med Mol Imag. 2017;45(3):392–401.
Grisanti F, Prieto E, Bastidas JF, Sancho L, Rodrigo P, Beorlegui C, et al. 3D voxel-based dosimetry to predict contralateral hypertrophy and an adequate future liver remnant after lobar radioembolization. Eur J Nucl Med Mol Imag. 2021;48(10):3048–57.
Spreafico C, Sposito C, Vaiani M, Cascella T, Bhoori S, Morosi C, et al. Development of a prognostic score to predict response to Yttrium-90 radioembolization for hepatocellular carcinoma with portal vein invasion. J Hepatol. 2018;68(4):724–32.
Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(1):17–29.
Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol boil Phys. 2011;79:163.
Gabr A, Riaz A, Johnson GE, Kim E, Padia S, Lewandowski RJ, et al. Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imag. 2021;48(2):580–3.
Lau WY, Kennedy AS, Kim YH, Lai HK, Lee RC, Leung TWT, et al. Patient selection and activity planning guide for selective internal radiotherapy with Yttrium-90 resin microspheres. Int J Radiat Oncol Biol Phys. 2012;82:401–7.
Levillain H, Bagni O, Deroose CM, Dieudonné A, Gnesin S, Grosser OS, et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur J Nucl Med Mol Imag. 2021;48(5):1570–84.
Weber M, Lam M, Chiesa C, Konijnenberg M, Cremonesi M, Flamen P, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds Writing group. Eur J Nucl Med Mol Imaging 2021, in press.
Funding
Not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Etienne Garin is consultant for Boston Scientific and reports receiving a grant, personal fees, and non-financial support from Boston Scientific. Boris Guiu is consultant for Boston Scientific declares that he has no conflict of interest. Julien Edeline reports receiving a grant from Boston Scientific during the conduct of the study; personal fees from Boston Scientific, Bayer, Roche, Eisai, Merck Sharpe & Dohme, AstraZeneca and Ipsen; grants and personal fees from Bristol Myers Squibb; and non-financial support from Amgen, outside the submitted work. Yan Rolland reports receiving a grant from Boston Scientific.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study, informed consent is not required.
Consent for Publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garin, E., Guiu, B., Edeline, J. et al. Trans-arterial Radioembolization Dosimetry in 2022. Cardiovasc Intervent Radiol 45, 1608–1621 (2022). https://doi.org/10.1007/s00270-022-03215-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03215-x