Abstract
Purpose
In light of recently published clinical reports and trials, the TheraSphere Global Dosimetry Steering Committee (DSC) reconvened to review new data and to update previously published clinical and dosimetric recommendations for the treatment of hepatocellular carcinoma (HCC).
Methods
The TheraSphere Global DSC is comprised of health care providers across multiple disciplines involved in the treatment of HCC with yttrium-90 (Y-90) glass microsphere–based transarterial radioembolization (TARE). Literature published between January 2019 and September 2021 was reviewed, discussed, and adjudicated by the Delphi method. Recommendations included in this updated document incorporate both the results of the literature review and the expert opinion and experience of members of the committee.
Results
Committee discussion and consensus led to the expansion of recommendations to apply to five common clinical scenarios in patients with HCC to support more individualized efficacious treatment with Y-90 glass microspheres. Existing clinical scenarios were updated to reflect recent developments in dosimetry approaches and broader treatment paradigms evolving for patients presenting with HCC.
Conclusion
Updated consensus recommendations are provided to guide clinical and dosimetric approaches for the use of Y-90 glass microsphere TARE in HCC, accounting for disease presentation, tumor biology, and treatment intent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, there has been an increasing use of transarterial radioembolization (TARE) using yttrium-90 (Y-90) glass microspheres in patients with hepatocellular carcinoma (HCC) across the Barcelona Clinic Liver Cancer (BCLC) classification spectrum. Since the previous publication of clinical and dosimetric considerations for TARE with Y-90 glass microspheres, additional published work has demonstrated the critical role of personalized dosimetry, optimizing dosing and patient selection in achieving improved clinical outcomes [1,2,3]. Additionally, several investigations demonstrated that increasing tumor-absorbed dose increases the likelihood of achieving complete pathologic necrosis, without compromising safety, while limiting toxicity by minimizing normal liver tissue exposure [4, 5]. These investigations have led to the inclusion of TARE in the updated BCLC staging system for treatment of single-HCC lesions ≤ 8 cm [6]. The decision to use single-compartment or multicompartment dosimetry in these studies underscores the importance of matching the appropriate TARE treatment approach with specific patient characteristics and treatment goals [1,2,3]. Given the number of new trials, recent publications, and their rapid impact on clinical practice, the TheraSphere Global DSC was reconvened to evaluate these new data and to update the recommendations [7].
Methods
The committee, comprised of interventional radiologists, radiation oncologists, nuclear medicine physicians, clinical scientists, medical oncologists, and physicists, reconvened for four 2-h virtual meetings, with an additional offline review in preparation for the process of updating the recommendations. During the first meeting, the committee reviewed the prior recommendations, recently published literature, and discussed updates. To identify literature since the prior recommendations, a PubMed search using a combination of the following search terms was conducted: transarterial radioembolization, TARE, brachytherapy, internal radiation therapy, SIRT, Y90, yttrium-90, TheraSphere, hepatocellular carcinoma, and HCC. The committee also considered whether new data warranted changing the degree of recommendation and/or the strength of consensus from the previous recommendations (Tables 1 and 2). Briefly, per the Delphi method, consensus was defined during virtual meetings as outlined in Table 2; strong disagreements by members were recorded and highlighted within the recommendation as caveats, where applicable. During the second and third meetings, committee members reviewed the revised recommendations and discussed each change collectively. Between the meetings, the lead author (RS) revised the recommendations based upon committee discussion and comments. The fourth and final meeting was a comprehensive review of the recommendations and a review of the draft manuscript. Steering committee members then had the opportunity to review and refine the manuscript independently, and final comments were incorporated into the manuscript by the lead author. All authors formally endorsed the manuscript and its recommendations prior to submission.
The reviewed literature included all published studies of TARE with glass microspheres for HCC since January 1, 2019; additional studies outside of this timeframe were reviewed if suggested by steering committee members. Publications addressing technical challenges rather than clinically oriented approaches were not included in the review. The recommendations included in this updated document incorporate both the critical literature review and the expert opinion and experience of members of the committee. All recommendations made in Tables 3, 4, 5, 6, and 7 are subject to regulatory and clinical standards within each country.
In light of the changing paradigms and treatment goals associated with Y-90 glass microsphere TARE, the committee agreed to expand the clinical scenarios from four to five, separating multifocal unilobar and bilobar disease recommendations, and to revise the definition of each. The scenarios subsequently addressed in the updated recommendations are as follows:
Curative intent:
-
Radiation segmentectomy: Localized disease (one or multiple tumors located in ≤ 2 segments), with contemporary and modern treatment approaches delivering superselectively to subsegments of liver, referred to as angiosomes (i.e., hepatic territory perfused by a specific branch of the hepatic artery), with the intent of delivering an ablative dose to tumor and normal tissue. Radiation segmentectomy no longer narrowly defined as ≤ 2 segments but rather inclusive of smaller hepatic segmentectomy
-
Radiation lobectomy: Unilobar disease, with the ultimate goal of disease control and contralateral lobar hypertrophy in the context of small future liver remnant (FLR), as a bridge to surgery (resection)
Palliative intent:
-
Multifocal unilobar disease without macrovascular invasion or portal vein thrombosis (MVI/PVT), with the goal of palliation and delay in progression; in select patients, intent may be conversion to curative options
-
Multifocal bilobar disease without MVI/PVT, with the goal of palliating and delaying progression, usually in combination or in sequence with systemic treatment
-
HCC with MVI/PVT, with the goal of palliating and delaying progression; in select patients, intent may be conversion to curative options
Key definitions used throughout this document were defined in the original publication and are reprinted below for ease of reference [7]:
-
Mean absorbed dose: Quantity is expressed in gray (Gy) in order to describe the average energy (J) deposited within a volume of interest (VOI) within a specific given mass (kg). The mean absorbed dose is referred to as “Dose” and is distinctly different than “Activity” or “Dosage” (GBq) [8, 9].
-
MIRD schema: The Medical Internal Radiation Dose (MIRD) schema is applicable to both the single-compartment and multicompartment dosimetry models. The mean absorbed dose (D) in any specific VOI (i.e., perfused volume, lobe, tumor or normal tissue) with mass of any VOI, denoted as M, with the assumption that D is distributed uniformly in each volume with permanent microsphere implantation and no biological clearance [10, 11]. Using this schema, D in a VOI is computed as:
where A is the net activity of 90Y implanted in the VOI, and F is the lung shunt fraction. As an example, if 2.2 GBq of glass microspheres was infused with a residual of 1% and a lung shunt of 5%, the net implanted activity in the liver tissue would be 2.2 × (0.99) × (0.95) = 2.07 GBq, and 2.07 GBq would represent the final activity within a MIRD formula for determining final tissue dose.
$${D}_{\left(\mathrm{Gy}\right)}=\frac{{A}_{(\mathrm{GBq})}\times ({50}_{(\mathrm{Gy}/\mathrm{kg}/\mathrm{GBq})}\left(1-F\right))}{{M}_{(\mathrm{kg})}}$$ -
Single-compartment model: A MIRD dosimetry model that assumes the 90Y microspheres (and therefore absorbed dose) are distributed uniformly within the VOI. In this model, only a uniform averaged D value over the VOI is calculated, without consideration of Y-90 activity distribution within the tumor and normal parenchyma. In reality, hypervascular tumors will absorb more microspheres and receive a higher dose, while the normal hepatic tissue will absorb fewer spheres and receive a lower dose [12,13,14].
-
Multicompartment model: A MIRD-based dosimetry approach where D is determined in more than one VOI, such as the tumor VOI and the normal parenchyma VOI. The lung also represents another compartment to which D can be estimated (based on a single-compartment model). Partition modeling refers to the multicompartment dosimetry approach reporting the tumoral and non-tumoral doses separately with a single averaged tumor to averaged non-tumoral uptake ratio (T:N ratio) [10].
Results
Relevant publications from the literature review, including additional suggestions by the committee, resulted in the inclusion of 31 new publications [1,2,3,4,5,6, 16, 17, 19, 20, 24,25,26, 28,29,30,31, 35, 36, 41, 47, 48, 50,51,52,53,54,55,56,57,58] and formed the basis for updates to the recommendations.
Clinical scenarios
Scenario 1: radiation segmentectomy for HCC
Radiation segmentectomy has been previously defined as the administration of Y-90 to ≤ 2 Couinaud segments with curative intent. In practical terms, this translates to subselective or superselective radioembolization. In most scenarios, a superselective, subsegmental infusion covering significantly ≤ 1 is achieved [15, 16]. For radiation segmentectomy, the percentage total liver volume that is treated should be considered. As long as a minimal amount of tissue is exposed, radiation segmentectomy may be considered in the setting of prior surgical resection (particularly if robust post-surgical hypertrophy has been observed). In the presence of stable but reduced hepatic function, radiation segmentectomy should be undertaken using caution and consideration of alternate options [3, 17,18,19]. Details are included in Table 3.
The objective is ablative dosing for both tumoral and non-tumoral compartments as both are expendable in this scenario [3]. The strength of these recommendations was an A in the previous version and remains an A with a strong degree of consensus from the committee; this recommendation was further reinforced by the findings of the prospective RASER study [20]. Partition dosimetry was not recommended by the panel in this scenario.
Scenario 2: radiation lobectomy for HCC
In unilobar HCC patients, resection is often not possible due to small FLR. For these patients, radiation lobectomy may be an option, as delivery of a high dose of radiation to one lobe may trigger the hypertrophy-atrophy complex, inducing hypertrophy in the contralateral lobe and thereby increasing the functional liver volume while controlling tumor growth in the treated lobe (Table 4) [21,22,23,24]. The increased FLR may allow for subsequent curative resection.
Depending on patient biology and mapping results, either single-compartment or multicompartment dosimetry approaches may be used [1, 21, 25, 26]. Hepatobiliary scintigraphy (HBS), including 99mTc-iminodiacetic acid (HIDA) or 99mTc-mebrofenin, and the use of Eovist/Primovist contrast media in magnetic resonance imaging are emerging investigational techniques that are being implemented for regional functional assessment of the FLR before and after Y-90 treatment. Additional data are needed to support a strong recommendation of their use as a standard of care in radiation lobectomy and resection planning [27,28,29]. The strength of these recommendations was previously a B with strong consensus from the committee; despite these additional studies and data, the committee deemed the data to be insufficient to raise the grade to A and maintained the grade of B with strong consensus.
Scenario 3: multifocal unilobar HCC without macrovascular invasion (MVI/PVT)
For patients with multifocal unilobar disease without MVI/PVT, radioembolization can be used for palliation and prevention of disease progression (Table 5). In this population, the goal should be to maximize tumor dose while preserving liver function. The committee recommends that radioembolization be used for Child–Pugh A patients and that a multidisciplinary tumor board review be conducted, especially for Child–Pugh B patients who have more severe hepatic dysfunction [1, 23, 30,31,32]. In select cases, this group of patients may be considered for resection if they exhibit responses and hypertrophy features of radiation lobectomy.
Previously, multifocal unilobar and bilobar diseases were grouped together and granted a B grade with a strong committee consensus; this newly created unilobar disease section has been given a grade of B with strong committee consensus.
Scenario 4: multifocal bilobar HCC without MVI/PVT
In multifocal bilobar HCC without MVI/PVT, the primary goals of treatment are frequently palliation, delaying disease progression, and sequencing with other liver-directed therapies and/or systemic treatment (Table 6). As with other clinical scenarios, the goal should be to maximize the dose of radiation delivered to the tumor while minimizing the dose to remaining normal liver parenchyma. Previously, multifocal unilobar and bilobar diseases were grouped together and given a grade of B with a strong committee consensus; this newly created bilobar disease section has been given a grade of B with strong committee consensus.
Scenario 5: HCC with MVI/PVT
Patients with portal vein thrombosis (MVI/PVT), indicative of advanced HCC, generally have a poor prognosis. Such patients are not usually considered transplantation or resection candidates and may not achieve optimal outcomes with chemoembolization [1, 33,34,35]. With careful patient selection and dosimetric planning, radioembolization may achieve a long-term durable response without compromise of hepatic function in this population (Table 7) [1, 34,35,36,37,38,39]. The committee recommended a shift in defining which patients with MVI/PVT should be selected for treatment with Y-90 glass microsphere TARE, narrowing from previous broader recommendations to those who are Child–Pugh A5 or A6 (except in the case of segmental MVI/PVT where radiation segmentectomy may be considered [1, 34,35,36]. Multicompartment dosimetry is preferred in these patients to ensure that the maximum tumor-absorbed dose (TAD) is achieved while minimizing the dose to the normal tissue–absorbed dose (NTAD) and allowing the assessment of MVI/PVT targeting evaluation during pretreatment planning [1, 35, 40]. As with the approaches discussed earlier, an adequately high specific activity (the amount of radioactivity per microsphere at the time of administration) is important to achieve a desired TAD in the MVI/PVT with potentially limited microsphere deposition [1]. Given data from this and other recent studies, the committee chose to increase the degree of recommendation from B to A, with a moderate degree of committee consensus.
Discussion
Results from over 30 manuscripts and abstracts published since 2019 prompted an update to treatment recommendations for Y-90 glass microsphere–based TARE in HCC patients; these included the DOSISPHERE-01, LEGACY, and TARGET studies [1,2,3]. While previous studies highlighted the improved overall survival in patients achieving complete response upon imaging, data from the recent DOSISPHERE-01 and TARGET studies further established associations between TAD, tumor response, and overall survival [1, 2]. For patients with limited disease, ablative Y-90 TARE remains the most effective and well-tolerated treatment option in eligible patients. Important new updates to the recommendations based on recent publications include more specific dosimetric recommendations for radiation segmentectomy and lobectomy, separating multifocal unilobar and bilobar diseases into different sets of recommendations and providing context in which Y-90 glass microsphere TARE should be used for patients with portal vein thrombosis. Additional multicompartment dosimetry updates included proposed new thresholds for tumor and NTAD and incorporated the impact of underlying liver function [3, 4, 41,42,43]. Multiple publications focused on the utility of 99mTc-MAA imaging to estimate Y-90 glass microsphere distribution confirm the distribution of treatment and whether to select tumor or NTAD as the primary driver in choosing the appropriate Y-90 activity using multicompartment dosimetry [1, 41, 43].
Ablative dosing approaches in radiation segmentectomy
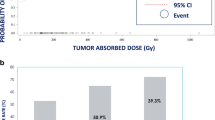
Recent publications, including LEGACY and its companion publications, have reported on higher selective treatment-absorbed doses for radiation segmentectomy [3, 4, 42]. Higher absorbed dose in selective ablative Y-90 glass microsphere TARE led to increased rates of complete pathologic necrosis, e.g., ≥ 400 Gy; complete and extensive pathologic necrosis have been shown to be associated with reduced recurrence in patients bridging to transplant [5, 42]. However, a maximum perfused volume-absorbed dose has not yet been identified. Recent publications also refined guidance for albumin-bilirubin (ALBI)-1/Child–Pugh A and ALBI-2/Child–Pugh B patients from up to 2 Couinaud segments to specific volume recommendations [17]. The use of cone-beam CT or angio-CT with selective intra-arterial contrast enhancement provides the best preprocedural volume definitions for accurate dosimetry calculations, provides the most accurate arterial flow assessing for non-target flow and coverage of microsatellites, and ensures dose target accuracy [19].
Improving conversion to resection
Recent studies and published recommendations have demonstrated the utility of personalized dosimetry in converting unresectable patients to candidates for resection [1, 3, 4, 21, 23, 25, 44]. Some recent publications showed that such an approach not only increased overall tumor response, but approximately doubled response rates when the TAD exceeded 300 Gy [1, 3]. In one of these investigations, multicompartment dosimetry used in multifocal unilobar HCC with or without MVI/PVT offered superior conversion of unresectable HCC compared to standard lobar single-compartment dosimetry (36% versus 4%, respectively) [1]. Single-compartment dosimetry using radiation lobectomy or modified lobectomy imparts local tumor control and contralateral lobe hypertrophy as a bridging strategy prior to resection. Collectively, these data demonstrated that treatment efficacy outcomes exhibit a continuum of improvement as TAD is escalated [3, 4, 21, 23]. Select studies provide guidance on the NTAD maximum and support evaluation of liver function, i.e., baseline bilirubin, prior to selecting NTAD targets [41]. Treatment outcome data regarding the use of NTAD to guide Y-90 glass microsphere activity selection are limited; further clinical data is needed to strengthen recommendations.
Differing treatment approaches for unilobar and bilobar HCC
Based on approvals of new systemic treatment options, the committee decided to divide unilobar and bilobar diseases without MVI/PVT into two separate clinical scenarios [30]. Systemic therapy is the current standard of care for advanced disease; however, it is important to consider TARE early in treatment planning, as it plays an important role in providing a cytotoxic effect, while ensuring tumor control, preserving liver function, and keeping future treatment options available [45, 46]. In these patients, multicompartment dosimetry is preferred to adequately assess TAD and NTAD relative to the extent of disease and liver function. Select centers have published Y-90 glass microsphere TARE experience in patients undergoing bilobar treatment, demonstrating that a multicompartment dosimetry approach is appropriate and beneficial in bilobar HCC [35, 43, 47].
The NTAD values proposed for unilobar treatment, 50–90 Gy based on baseline bilirubin levels, are higher than those recommended for bilobar patients, 40–70 Gy. However, additional clinical data are needed to better define the appropriate range for bilobar patients [41, 43]. In the case that multicompartment dosimetry is not feasible and single-compartment dosimetry is used, a lower target (i.e., 120 Gy to the perfused volume) is recommended for bilobar HCC patients; planning for such treatment should include careful evaluation of clinical status and liver function when evaluating possible treatment options. A more conservative approach to TAD and NTAD is recommended in the palliative setting as compared to in patients where treatment intent includes downstaging or conversion to resection.
Treatment goals for HCC with MVI/PVT
In HCC patients with MVI/PVT, treatment goals (i.e., potential downstaging or conversion to resection) and careful patient selection should drive the decision as to whether TARE should be used as monotherapy or in conjunction with systemic treatment. In both DOSISPHERE-01 and TARGET, Y-90 glass microsphere TARE was evaluated in patients with MVI/PVT as monotherapy [1, 2]. Patients in DOSISPHERE-01 were selected based on a dual targeting of tumor and MVI/PVT, crucial to tumor control and resolution of MVI/PVT [1]. Over 40% of patients with MVI/PVT in DOSISPHERE-01 who were treated with multicompartment dosimetry were converted to resection, compared to no patients in the single-compartment dosimetry arm. In the presence of ongoing cirrhotic liver function decline, systemic agents may be considered early on in treatment and in combination with Y-90 following a multidisciplinary review; however, further investigation is needed to better determine specific timing and treatment algorithms [48, 49]. Multicompartment dosimetry is the preferred treatment option in this patient population.
Future directions
As noted throughout the recommendations, there remain several areas which require additional investigations to better understand optimized patient selection and outcomes. The investigational research areas identified by the committee as high interest and requiring additional data and publications to inform clinical practice are detailed in Table 8. The committee encourages the collection and publication of clinical data to further provide evidence relating to the updated recommendations in these key areas of Y-90 glass microsphere TARE for HCC patients. Here, we briefly discuss two of these areas in which research is currently being or was recently conducted: the use of functional assessments in treatment planning and increasing reproducibility of dosimetric approaches.
Functional assessments in treatment planning
In a parallel advancement in treatment planning for Y-90 glass microsphere TARE patients with insufficient FLR, functional assessments have been proposed in addition to volume assessments. Traditionally, the timing to undergo resection has focused solely on hypertrophy of the contralateral lobe; however, mounting evidence suggests a role for hepatobiliary scintigraphy to assess regional adequate liver function in confirming treatment candidacy [27,28,29]. Though the time course of function and volume recovery are parallel, functional recovery lags behind volume. Functional assessment may better drive key clinical decisions regarding treatment success, such as follow-up duration, the need for additional Y-90 treatment, and surgical timing or additional observations. Additional investigation is necessary to confirm the utility of functional assessment in making such treatment decisions.
Variability and reproducibility of dosimetry
One oft-cited concern with using more complex dosimetry-based approaches is the prediction reproducibility in the treatment phase; however, there are now several tools available to help address these challenges. Several commercial dosimetry software options are available, streamlining calculation of multicompartment dosimetry. Advancements in catheter technique in addition to refinement of the use of 99mTc-MAA imaging to estimate Y-90 glass microsphere distribution have led to improved clinical utility [50,51,52,53]. NTAD exhibits better reproducibility, which provides confidence in its use as a safety threshold. Although reproducibility in TAD may be suboptimal, it has been shown to be predictive of patient outcomes (such as tumor response and overall survival) in DOSISPHERE-01, TARGET, and other recent single-center publications [1, 2, 50, 51, 53]. To date, publications evaluated all patients to ascertain reproducibility. However, it may be appropriate to screen out patients in whom multicompartment tumor dosimetry predictions may be unreliable; in those cases, defaulting to MIRD is recommended. Recent publications note that variability may be driven by a limited sample size of published data, operator experience, and variability of tumor flow (and hence T:N) in the presence of multifocal disease, suggesting further optimization of patient selection is needed to improve accuracy and reproducibility of multicompartment dosimetry [52].
Conclusions
While Y-90 glass microsphere TARE is a key tool in the HCC treatment arsenal, appropriate patient selection, multidisciplinary review, and consideration of alternative or combination treatment in the algorithm are critical to achieving optimal patient outcomes. A number of advancements have been incorporated into the updated HCC treatment recommendations for Y-90 glass microsphere TARE presented here in an effort to improve patient selection, toxicity profiles, and outcomes.
Data availability
All data reviewed in the creation of these recommendations can be found in the published literature, per the manuscript’s bibliography.
Abbreviations
- ALBI:
-
Albumin-bilirubin
- BCLC:
-
Barcelona Clinic Liver Cancer
- CT:
-
Computed tomography
- FLR:
-
Future liver remnant
- HBS:
-
Hepatobiliary scintigraphy
- HCC:
-
Hepatocellular carcinoma
- HIDA:
-
Hepatobiliary iminodiacetic acid
- MR:
-
Magnetic resonance
- MVI/PVT:
-
Macrovascular invasion/portal vein thrombosis
- NTAD:
-
Normal tissue–absorbed dose
- TAD:
-
Tumor-absorbed dose
- TARE:
-
Transarterial radioembolization
- Y-90:
-
Yttrium-90
References
Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. The Lancet Gastroenterology & hepatology. 2021;6:17–29. https://doi.org/10.1016/s2468-1253(20)30290-9.
Lam M, Garin E, Maccauro M, Kappadath SC, Sze D, Turkmen C, et al. A Global Evaluation of advanced dosimetry in transarterial radioembolization of hepatocellular carcinoma with yttrium-90: the TARGET Study. European Journal of Nuclear Medicine and Molecular Imaging. 2022;in press.
Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY Study. Hepatology (Baltimore, Md). 2021. doi:https://doi.org/10.1002/hep.31819.
Gabr A, Riaz A, Johnson GE, Kim E, Padia S, Lewandowski RJ, et al. Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imaging. 2021;48:580–3. https://doi.org/10.1007/s00259-020-04976-8.
Gabr A, Kulik L, Mouli S, Riaz A, Ali R, Desai K, et al. Liver transplantation following yttrium-90 radioembolization: 15-year experience in 207-patient cohort. Hepatology (Baltimore, MD). 2021;73:998–1010. https://doi.org/10.1002/hep.31318.
Reig M, Forner A, Rimola J, Ferrer-Fábrega J, Burrel M, Garcia-Criado A, et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system. The 2022 update. J Hepatol. 2021. https://doi.org/10.1016/j.jhep.2021.11.018.
Salem R, Padia SA, Lam M, Bell J, Chiesa C, Fowers K, et al. Clinical and dosimetric considerations for Y90: recommendations from an international multidisciplinary working group. Eur J Nucl Med Mol Imaging. 2019;46:1695–704. https://doi.org/10.1007/s00259-019-04340-5.
Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet no. 21: a generalized schema for radiopharmaceutical dosimetry--standardization of nomenclature. J Nucl Med. 2009;50:477–84. https://doi.org/10.2967/jnumed.108.056036.
Report 85: fundamental quantities and units for ionizing radiation. J ICRU. 2011;11:1–31. https://doi.org/10.1093/jicru/ndr011.
Gulec SA, Mesoloras G, Stabin M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: the MIRD equations for dose calculations. J Nucl Med. 2006;47:1209–11.
Dezarn WA, Cessna JT, DeWerd LA, Feng W, Gates VL, Halama J, et al. Recommendations of the American Association of Physicists in Medicine on dosimetry, imaging, and quality assurance procedures for 90Y microsphere brachytherapy in the treatment of hepatic malignancies. Med Phys. 2011;38:4824–45. https://doi.org/10.1118/1.3608909.
Salem R, Thurston KG. Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 2: special topics. Journal of Vascular and Interventional Radiology: JVIR. 2006;17:1425–39. https://doi.org/10.1097/01.Rvi.0000235779.88652.53.
Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. Journal of Vascular and Interventional Radiology: JVIR. 2006;17:1251–78. https://doi.org/10.1097/01.Rvi.0000233785.75257.9a.
Salem R, Thurston KG, Carr BI, Goin JE, Geschwind JF. Yttrium-90 microspheres: radiation therapy for unresectable liver cancer. Journal of Vascular and Interventional Radiology: JVIR. 2002;13:S223–9. https://doi.org/10.1016/s1051-0443(07)61790-4.
Padia SA, Johnson GE, Horton KJ, Ingraham CR, Kogut MJ, Kwan S, et al. Segmental yttrium-90 radioembolization versus segmental chemoembolization for localized hepatocellular carcinoma: results of a single-center, retrospective, propensity score-matched study. Journal of Vascular and Interventional Radiology: JVIR. 2017;28:777-85.e1. https://doi.org/10.1016/j.jvir.2017.02.018.
Gabr A, Ranganathan S, Mouli SK, Riaz A, Gates VL, Kulik L, et al. Streamlining radioembolization in UNOS T1/T2 hepatocellular carcinoma by eliminating lung shunt estimation. J Hepatol. 2020;72:1151–8. https://doi.org/10.1016/j.jhep.2020.02.024.
De la Garza-Ramos C, Overfield CJ, Montazeri SA, Liou H, Paz-Fumagalli R, Frey GT, et al. Biochemical safety of ablative yttrium-90 radioembolization for hepatocellular carcinoma as a function of percent liver treated. J Hepatocell Carcinoma. 2021;8:861–70. https://doi.org/10.2147/jhc.S319215.
Louie JD, Kothary N, Kuo WT, Hwang GL, Hofmann LV, Goris ML, et al. Incorporating cone-beam CT into the treatment planning for yttrium-90 radioembolization. Journal of Vascular and Interventional Radiology: JVIR. 2009;20:606–13. https://doi.org/10.1016/j.jvir.2009.01.021.
Stein SI, Soliman MM, Sparapani J, Doustaly R, Cobb BW, Malhotra A, et al. Conventional hepatic volumetry may lead to inaccurate segmental yttrium-90 radiation dosimetry. Cardiovasc Intervent Radiol. 2021. https://doi.org/10.1007/s00270-021-02898-y.
Kim E, Sher A, Abboud G, Schwartz M, Facciuto M, Tabrizian P, et al. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): a single-centre, single-arm study. Lancet Gastroenterol Hepatol. 2022. https://doi.org/10.1016/S2468-1253(22)00091-7
Gabr A, Riaz A, Mouli S, Desai K, Thornburg B, Salem R, et al. Modified radiation lobectomy: an evolving paradigm to convert patients to liver resection candidacy. Semin Interv Radiol. 2019;36:343–8. https://doi.org/10.1055/s-0039-1696648.
Ahmed A, Stauffer JA, LeGout JD, Burns J, Croome K, Paz-Fumagalli R, et al. The use of neoadjuvant lobar radioembolization prior to major hepatic resection for malignancy results in a low rate of post hepatectomy liver failure. Journal of Gastrointestinal Oncology. 2021;12:751–61. https://doi.org/10.21037/jgo-20-507.
Gabr A, Polineni P, Mouli SK, Riaz A, Lewandowski RJ, Salem R. Neoadjuvant Radiation Lobectomy As an Alternative to Portal Vein Embolization in Hepatocellular Carcinoma. Semin Nucl Med. 2019;49:197–203. https://doi.org/10.1053/j.semnuclmed.2019.01.009.
Bekki Y, Marti J, Toshima T, Lewis S, Kamath A, Argiriadi P, et al. A comparative study of portal vein embolization versus radiation lobectomy with yttrium-90 micropheres in preparation for liver resection for initially unresectable hepatocellular carcinoma. Surgery. 2021;169:1044–51. https://doi.org/10.1016/j.surg.2020.12.012.
Palard X, Edeline J, Rolland Y, Le Sourd S, Pracht M, Laffont S, et al. Dosimetric parameters predicting contralateral liver hypertrophy after unilobar radioembolization of hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2018;45:392–401. https://doi.org/10.1007/s00259-017-3845-7.
Chiesa C, Sjogreen-Gleisner K, Walrand S, Strigari L, Flux G, Gear J, et al. EANM dosimetry committee series on standard operational procedures: a unified methodology for (99m)Tc-MAA pre- and (90)Y peri-therapy dosimetry in liver radioembolization with (90)Y microspheres. EJNMMI Physics. 2021;8:77. https://doi.org/10.1186/s40658-021-00394-3.
Allimant C, Deshayes E, Kafrouni M, Santoro L, de Verbizier D, Fourcade M, et al. Hepatobiliary scintigraphy and glass (90)Y radioembolization with personalized dosimetry: dynamic changes in treated and nontreated liver. Diagnostics (Basel, Switzerland). 2021;11. https://doi.org/10.3390/diagnostics11060931.
van der Velden S, Braat M, Labeur TA, Scholten MV, van Delden OM, Bennink RJ, et al. A pilot study on hepatobiliary scintigraphy to monitor regional liver function in (90)Y radioembolization. J Nucl Med. 2019;60:1430–6. https://doi.org/10.2967/jnumed.118.224394.
Syed M, Shah J, Montazeri SA, Grajo JR, Geller B, Toskich B. Analysis of dynamic hepatobiliary contrast-enhanced MRI signal intensity after yttrium-90 radioembolization with glass microspheres for the treatment of hepatocellular carcinoma. Abdominal Radiology (New York). 2021;46:2182–7. https://doi.org/10.1007/s00261-020-02855-2.
Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology (Baltimore, MD). 2018;68:1429–40. https://doi.org/10.1002/hep.29691.
Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. https://doi.org/10.1053/j.gastro.2009.09.006.
Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology (Baltimore, MD). 2013;57:1826–37. https://doi.org/10.1002/hep.26014.
Gorodetski B, Chapiro J, Schernthaner R, Duran R, Lin M, Lee H, et al. Advanced-stage hepatocellular carcinoma with portal vein thrombosis: conventional versus drug-eluting beads transcatheter arterial chemoembolization. Eur Radiol. 2017;27:526–35. https://doi.org/10.1007/s00330-016-4445-9.
Abouchaleh N, Gabr A, Ali R, Al Asadi A, Mora RA, Kallini JR, et al. (90)Y radioembolization for locally advanced hepatocellular carcinoma with portal vein thrombosis: long-term outcomes in a 185-patient cohort. J Nucl Med. 2018;59:1042–8. https://doi.org/10.2967/jnumed.117.199752.
Ho CL, Chen S, Cheung SK, Leung YL, Cheng KC, Wong KN, et al. Radioembolization with (90)Y glass microspheres for hepatocellular carcinoma: significance of pretreatment (11)C-acetate and (18)F-FDG PET/CT and posttreatment (90)Y PET/CT in individualized dose prescription. Eur J Nucl Med Mol Imaging. 2018;45:2110–21. https://doi.org/10.1007/s00259-018-4064-6.
Memon K, Kulik L, Lewandowski RJ, Mulcahy MF, Benson AB, Ganger D, et al. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58:73–80. https://doi.org/10.1016/j.jhep.2012.09.003.
Ahn JC, Lauzon M, Luu M, Friedman ML, Kosari K, Nissen N, et al. Transarterial radioembolization versus systemic treatment for hepatocellular carcinoma with macrovascular invasion: analysis of the US National Cancer Database. J Nucl Med. 2021. https://doi.org/10.2967/jnumed.121.261954.
Cardarelli-Leite L, Chung J, Klass D, Marquez V, Chou F, Ho S, et al. Ablative transarterial radioembolization improves survival in patients with HCC and portal vein tumor thrombus. Cardiovasc Intervent Radiol. 2020;43:411–22. https://doi.org/10.1007/s00270-019-02404-5.
Garin E, Rolland Y, Pracht M, Le Sourd S, Laffont S, Mesbah H, et al. High impact of macroaggregated albumin-based tumour dose on response and overall survival in hepatocellular carcinoma patients treated with (90) Y-loaded glass microsphere radioembolization. Liver International: official journal of the International Association for the Study of the Liver. 2017;37:101–10. https://doi.org/10.1111/liv.13220.
Garin E, Rolland Y, Edeline J, Icard N, Lenoir L, Laffont S, et al. Personalized dosimetry with intensification using 90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J Nucl Med. 2015;56:339–46. https://doi.org/10.2967/jnumed.114.145177.
Chiesa C, Mira M, Bhoori S, Bormolini G, Maccauro M, Spreafico C, et al. Radioembolization of hepatocarcinoma with (90)Y glass microspheres: treatment optimization using the dose-toxicity relationship. Eur J Nucl Med Mol Imaging. 2020;47:3018–32. https://doi.org/10.1007/s00259-020-04845-4.
Toskich B, Vidal LL, Olson MT, Lewis JT, LeGout JD, Sella DM, et al. Pathologic response of hepatocellular carcinoma treated with yttrium-90 glass microsphere radiation segmentectomy prior to liver transplantation: a validation study. Journal of Vascular and Interventional Radiology: JVIR. 2021;32:518-26.e1. https://doi.org/10.1016/j.jvir.2020.12.019.
Thomas MA, Mahvash A, Abdelsalam M, Kaseb AO, Kappadath SC. Planning dosimetry for (90) Y radioembolization with glass microspheres: evaluating the fidelity of (99m) Tc-MAA and partition model predictions. Med Phys. 2020;47:5333–42. https://doi.org/10.1002/mp.14452.
Baker T, Tabrizian P, Zendejas I, Gamblin TC, Kazimi M, Boudjema K, et al. Conversion to resection post radioembolization in patients with HCC: recommendations from a multidisciplinary working group. HPB (Oxford). 2021. https://doi.org/10.1016/j.hpb.2021.12.013.
Teyateeti A, Mahvash A, Long JP, Abdelsalam ME, Avritscher R, Chasen B, et al. Survival outcomes for yttrium-90 transarterial radioembolization with and without sorafenib for unresectable hepatocellular carcinoma patients. J Hepatocell Carcinoma. 2020;7:117–31. https://doi.org/10.2147/jhc.S248314.
Kaseb AO, Kappadath SC, Lee SS, Raghav KP, Mohamed YI, Xiao L, et al. A prospective phase II study of safety and efficacy of sorafenib followed by (90)Y glass microspheres for patients with advanced or metastatic hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:1129–45. https://doi.org/10.2147/jhc.S318865.
Kappadath SC, Mikell J, Balagopal A, Baladandayuthapani V, Kaseb A, Mahvash A. Hepatocellular carcinoma tumor dose response after (90)Y-radioembolization with glass microspheres using (90)Y-SPECT/CT-based voxel dosimetry. Int J Radiat Oncol Biol Phys. 2018;102:451–61. https://doi.org/10.1016/j.ijrobp.2018.05.062.
Zhan C, Ruohoniemi D, Shanbhogue KP, Wei J, Welling TH, Gu P, et al. Safety of combined yttrium-90 radioembolization and immune checkpoint inhibitor immunotherapy for hepatocellular carcinoma. Journal of Vascular and Interventional Radiology: JVIR. 2020;31:25–34. https://doi.org/10.1016/j.jvir.2019.05.023.
Javan H, Dayyani F, Abi-Jaoudeh N. Therapy in advanced hepatocellular carcinoma. Semin Interv Radiol. 2020;37:466–74. https://doi.org/10.1055/s-0040-1719187.
Jadoul A, Bernard C, Lovinfosse P, Gérard L, Lilet H, Cornet O, et al. Comparative dosimetry between (99m)Tc-MAA SPECT/CT and (90)Y PET/CT in primary and metastatic liver tumors. Eur J Nucl Med Mol Imaging. 2020;47:828–37. https://doi.org/10.1007/s00259-019-04465-7.
Kafrouni M, Allimant C, Fourcade M, Vauclin S, Guiu B, Mariano-Goulart D, et al. Analysis of differences between (99m)Tc-MAA SPECT- and (90)Y-microsphere PET-based dosimetry for hepatocellular carcinoma selective internal radiation therapy. EJNMMI Res. 2019;9:62. https://doi.org/10.1186/s13550-019-0533-6.
Meyers N, Jadoul A, Bernard C, Delwaide J, Lamproye A, Detry O, et al. Inter-observer variability of (90)Y PET/CT dosimetry in hepatocellular carcinoma after glass microspheres transarterial radioembolization. EJNMMI Physics. 2020;7:29. https://doi.org/10.1186/s40658-020-00302-1.
d’Abadie P, Walrand S, Hesse M, Annet L, Borbath I, Van den Eynde M, et al. Prediction of tumor response and patient outcome after radioembolization of hepatocellular carcinoma using 90Y-PET-computed tomography dosimetry. Nucl Med Commun. 2021;42:747–54. https://doi.org/10.1097/mnm.0000000000001395.
Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163–71. https://doi.org/10.1016/j.ijrobp.2009.10.062.
Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology (Baltimore, MD). 2014;60:192–201. https://doi.org/10.1002/hep.27057.
Biederman DM, Titano JJ, Bishay VL, Durrani RJ, Dayan E, Tabori N, et al. Radiation segmentectomy versus TACE combined with microwave ablation for unresectable solitary hepatocellular carcinoma up to 3 cm: a propensity score matching study. Radiology. 2017;283:895–905. https://doi.org/10.1148/radiol.2016160718.
Biederman DM, Titano JJ, Korff RA, Fischman AM, Patel RS, Nowakowski FS, et al. Radiation segmentectomy versus selective chemoembolization in the treatment of early-stage hepatocellular carcinoma. Journal of Vascular and Interventional Radiology: JVIR. 2018;29:30-7.e2. https://doi.org/10.1016/j.jvir.2017.08.026.
Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, et al. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology. 2018;287:1050–8. https://doi.org/10.1148/radiol.2018171768.
Ali R, Riaz A, Gabr A, Abouchaleh N, Mora R, Al Asadi A, et al. Clinical outcomes of Y90 radioembolization for recurrent hepatocellular carcinoma following curative resection. Eur J Nucl Med Mol Imaging. 2017;44:2195–202. https://doi.org/10.1007/s00259-017-3792-3.
Gates VL, Hickey R, Marshall K, Williams M, Salzig K, Lewandowski RJ, et al. Gastric injury from (90)Y to left hepatic lobe tumors adjacent to the stomach: fact or fiction? Eur J Nucl Med Mol Imaging. 2015;42:2038–44. https://doi.org/10.1007/s00259-015-3122-6.
Atassi B, Bangash AK, Bahrani A, Pizzi G, Lewandowski RJ, Ryu RK, et al. Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics: a review publication of the Radiological Society of North America, Inc. 2008;28:81–99. https://doi.org/10.1148/rg.281065721.
Molvar C, Lewandowski R. Yttrium-90 radioembolization of hepatocellular carcinoma-performance, technical advances, and future concepts. Semin Interv Radiol. 2015;32:388–97. https://doi.org/10.1055/s-0035-1564704.
Hamoui N, Minocha J, Memon K, Sato K, Ryu R, Salem R, et al. Prophylactic embolization of the gastroduodenal and right gastric arteries is not routinely necessary before radioembolization with glass microspheres. Journal of Vascular and Interventional Radiology: JVIR. 2013;24:1743–5. https://doi.org/10.1016/j.jvir.2013.07.011.
Gabr A, Kallini JR, Gates VL, Hickey R, Kulik L, Desai K, et al. Same-day (90)Y radioembolization: implementing a new treatment paradigm. Eur J Nucl Med Mol Imaging. 2016;43:2353–9. https://doi.org/10.1007/s00259-016-3438-x.
Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Nemcek AA Jr, Kulik L, et al. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol. 2007;30:571–92. https://doi.org/10.1007/s00270-007-9064-z.
Memon K, Kulik L, Lewandowski RJ, Wang E, Riaz A, Ryu RK, et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology. 2011;141(526–35):35.e1-2. https://doi.org/10.1053/j.gastro.2011.04.054.
Riaz A, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora R, et al. Radioembolization for hepatocellular carcinoma: Statistical confirmation of improved survival in responders by landmark analyses. Hepatology (Baltimore, MD). 2018;67:873–83. https://doi.org/10.1002/hep.29480.
Spreafico C, Maccauro M, Mazzaferro V, Chiesa C. The dosimetric importance of the number of 90Y microspheres in liver transarterial radioembolization (TARE). Eur J Nucl Med Mol Imaging. 2014;41:634–8. https://doi.org/10.1007/s00259-013-2674-6.
Vouche M, Lewandowski RJ, Atassi R, Memon K, Gates VL, Ryu RK, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59:1029–36. https://doi.org/10.1016/j.jhep.2013.06.015.
Edeline J, Lenoir L, Boudjema K, Rolland Y, Boulic A, Le Du F, et al. Volumetric changes after (90)y radioembolization for hepatocellular carcinoma in cirrhosis: an option to portal vein embolization in a preoperative setting? Ann Surg Oncol. 2013;20:2518–25. https://doi.org/10.1245/s10434-013-2906-9.
Vouche M, Degrez T, Bouazza F, Delatte P, Galdon MG, Hendlisz A, et al. Sequential tumor-directed and lobar radioembolization before major hepatectomy for hepatocellular carcinoma. World J Hepatol. 2017;9:1372–7. https://doi.org/10.4254/wjh.v9.i36.1372.
Matsumoto MM, Mouli S, Saxena P, Gabr A, Riaz A, Kulik L, et al. Comparing real world, personalized, multidisciplinary tumor board recommendations with BCLC algorithm: 321-patient analysis. Cardiovasc Intervent Radiol. 2021;44:1070–80. https://doi.org/10.1007/s00270-021-02810-8.
Chan KT, Alessio AM, Johnson GE, Vaidya S, Kwan SW, Monsky W, et al. Prospective trial using internal pair-production positron emission tomography to establish the yttrium-90 radioembolization dose required for response of hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2018;101:358–65. https://doi.org/10.1016/j.ijrobp.2018.01.116.
Chiesa C, Maccauro M, Romito R, Spreafico C, Pellizzari S, Negri A, et al. Need, feasibility and convenience of dosimetric treatment planning in liver selective internal radiation therapy with (90)Y microspheres: the experience of the National Tumor Institute of Milan. The Quarterly Journal of Nuclear Medicine and Molecular Imaging: official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the So. 2011;55:168–97.
Haste P, Tann M, Persohn S, LaRoche T, Aaron V, Mauxion T, et al. Correlation of technetium-99m macroaggregated albumin and yttrium-90 glass microsphere biodistribution in hepatocellular carcinoma: a retrospective review of pretreatment single photon emission CT and posttreatment positron emission tomography/CT. Journal of Vascular and Interventional Radiology: JVIR. 2017;28:722-30.e1. https://doi.org/10.1016/j.jvir.2016.12.1221.
Balagopal A, Kappadath SC. Characterization of (90) Y-SPECT/CT self-calibration approaches on the quantification of voxel-level absorbed doses following (90) Y-microsphere selective internal radiation therapy. Med Phys. 2018;45:875–83. https://doi.org/10.1002/mp.12695.
Chiesa C, Mira M, Maccauro M, Spreafico C, Romito R, Morosi C, et al. Radioembolization of hepatocarcinoma with (90)Y glass microspheres: development of an individualized treatment planning strategy based on dosimetry and radiobiology. Eur J Nucl Med Mol Imaging. 2015;42:1718–38. https://doi.org/10.1007/s00259-015-3068-8.
Seidensticker R, Seidensticker M, Damm R, Mohnike K, Schütte K, Malfertheiner P, et al. Hepatic toxicity after radioembolization of the liver using (90)Y-microspheres: sequential lobar versus whole liver approach. Cardiovasc Intervent Radiol. 2012;35:1109–18. https://doi.org/10.1007/s00270-011-0295-7.
Garin E, Rolland Y, Laffont S, Edeline J. Clinical impact of (99m)Tc-MAA SPECT/CT-based dosimetry in the radioembolization of liver malignancies with (90)Y-loaded microspheres. Eur J Nucl Med Mol Imaging. 2016;43:559–75. https://doi.org/10.1007/s00259-015-3157-8.
Acknowledgements
We thank Evelyn Schnuerer, MSc. (Boston Scientific Corporation), Alexandra J. Greenberg-Worisek, PhD, MPH (Boston Scientific Corporation), and Paginae Incorporated, funded by Boston Scientific for medical writing assistance.
Funding
Author consultants were paid by Boston Scientific and received remuneration for time spent on this work. The work is under the sole responsibility the authors and does not represent the views or opinions of Boston Scientific Corporation.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the recommendations, contributed relevant data, participated in drafting, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Riad Salem is a consultant for Boston Scientific, AstraZeneca, Genentech, Sirtex, Cook, Eisai, Bard, and QED Therapeutics.
Siddharth Padia is a consultant for Boston Scientific Corporation, Teleflex Medical, Guerbet, Varian Medical Systems, and Johnson and Johnson.
Marnix Lam is a consultant for Boston Scientific, Terumo, and Quirem, and he receives research support from Boston Scientific, Terumo, Quirem, and Novartis.
Carlo Chiesa is a consultant for Terumo and Boston Scientific, and he received a research grant in 2017 from the latter company.
Paul Haste is a consultant for Boston Scientific.
Bruno Sangro received consulting fees from Adaptimmune, Astra-Zeneca, Bayer, BMS, Boston Scientific, Eisai, Exelixis, Eli-Lilly, IPSEN, Merck, Onxeo, Roche, Sirtex, and Terumo; lecture fees from Astra-Zeneca, Bayer, BMS, Eisai, Eli-Lilly, Incyte, IPSEN, Roche, and Sirtex; and institutional research grants from BMS and Sirtex.
Beau Toskich is a consultant for AstraZeneca, Genentech, Eisai, Boston Scientific, Sitrex Medical, Turnstone Biologics, Johnson and Johnson, HistoSonics, and VIVOS.
Kirk Fowers is an employee of Boston Scientific Corporation.
Joseph M. Herman is a consultant for Boston Scientific and HistoSonics and received institutional support for the Canopy Cancer Collective Learning Health Network.
S. Cheenu Kappadath has been a consultant for Boston Scientific, Sirtex Medical, ABK Biomedical, and Terumo Medical.
Thomas Leung is a consultant for Boston Scientific, SIRTEX, AstraZeneca, Eisai, and Ipsen.
Daniel Y. Sze was a consultant for Argon, Artio Medical, Astra-Zeneca, Bayer, BlackSwan Vascular, Boston Scientific, Bristol-Myers Squibb, Eisai, FluidX, W. L. Gore, Guerbet, Koli, RadiAction, Sirtex, Terumo, TriSalus Life Sciences, and Varian; received institutional research support from Boston Scientific, Merit Medical, and Sirtex; and serves on Independent Data Safety Monitoring Boards for W. L. Gore and Replimune.
Edward Kim is an advisory board member, speaker, and consultant for Boston Scientific; a consultant for Bristol-Myers Squibb; and an advisory board member for Genentech and Eisai.
Etienne Garin is a consultant for Boston Scientific.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Dosimetry
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salem, R., Padia, S.A., Lam, M. et al. Clinical, dosimetric, and reporting considerations for Y-90 glass microspheres in hepatocellular carcinoma: updated 2022 recommendations from an international multidisciplinary working group. Eur J Nucl Med Mol Imaging 50, 328–343 (2023). https://doi.org/10.1007/s00259-022-05956-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05956-w