Abstract
Purpose
To determine whether histotripsy can create human-scale transcostal ablations in porcine liver without causing severe thermal wall injuries along the beam path.
Materials and Methods
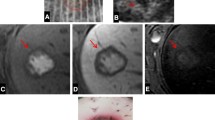
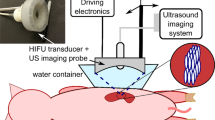
Histotripsy was applied to the liver using a preclinical prototype robotic system through a transcostal window in six female swine. A 3.0 cm spherical ablation zone was prescribed. Duration of treatment (75 min) was longer than a prior subcostal treatment study (24 min, 15 s) to minimize beam path heating. Animals then underwent contrast-enhanced MRI, necropsy, and histopathology. Images and tissue were analyzed for ablation zone size, shape, completeness of necrosis, and off-target effects.
Results
Ablation zones demonstrated complete necrosis with no viable tissue remaining in 6/6 animals by histopathology. Ablation zone volume was close to prescribed (13.8 ± 1.8 cm3 vs. prescribed 14.1 cm3). Edema was noted in the body wall overlying the ablation on T2 MRI in 5/5 (one animal did not receive MRI), though there was no gross or histologic evidence of injury to the chest wall at necropsy. At gross inspection, lung discoloration in the right lower lobe was present in 5/6 animals (mean size: 1 × 2 × 4 cm) with alveolar hemorrhage, preservation of blood vessels and bronchioles, and minor injuries to pneumocytes noted at histology.
Conclusion
Transcostal hepatic histotripsy ablation appears feasible, effective, and no severe injuries were identified in an acute porcine model when prolonged cooling time is added to minimize body wall heating.






Similar content being viewed by others
References
Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16:192–200. https://doi.org/10.1053/j.tvir.2013.08.002.
Poon RT, Ng KK, Lam CM, et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441–9.
Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–51. https://doi.org/10.1148/radiol.2262012198.
Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol. 2006;32:115–29. https://doi.org/10.1016/j.ultrasmedbio.2005.09.005.
Xu Z, Ludomirsky A, Eun LY, et al. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:726–36.
Vlaisavljevich E, Lin K-W, Maxwell A, et al. Effects of Ultrasound Frequency and Tissue Stiffness on the Histotripsy Intrinsic Threshold for Cavitation. Ultrasound Med Biol. 2015;41:1651–67. https://doi.org/10.1016/j.ultrasmedbio.2015.01.028.
Smolock AR, Cristescu MM, Vlaisavljevich E, et al. Robotically Assisted Sonic Therapy as a Noninvasive Nonthermal Ablation Modality: Proof of Concept in a Porcine Liver Model. Radiology. 2018;287:485–93. https://doi.org/10.1148/radiol.2018171544.
Longo KC, Knott EA, Watson RF, et al. Robotically Assisted Sonic Therapy (RAST) for Noninvasive Hepatic Ablation in a Porcine Model: Mitigation of Body Wall Damage with a Modified Pulse Sequence. Cardiovasc Intervent Radiol. 2019;42:1016–23. https://doi.org/10.1007/s00270-019-02215-8.
Lundt JE, Allen SP, Shi J, et al. Non-invasive, Rapid Ablation of Tissue Volume Using Histotripsy. Ultrasound Med Biol. 2017;43:2834–47. https://doi.org/10.1016/j.ultrasmedbio.2017.08.006.
Knott EAEA, Swietlik JFJF, Longo KCKC, et al. Robotically-Assisted Sonic Therapy for Renal Ablation in a Live Porcine Model: Initial Preclinical Results. J Vasc Interv Radiol. 2019;30:1293–302. https://doi.org/10.1016/j.jvir.2019.01.023.
Vlaisavljevich E, Kim Y, Allen S, et al. Image-guided non-invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model. Ultrasound Med Biol. 2013;39:1398–409. https://doi.org/10.1016/j.ultrasmedbio.2013.02.005.
Roberts WW, Hall TL, Ives K, et al. Pulsed Cavitational Ultrasound: A Noninvasive Technology for Controlled Tissue Ablation (Histotripsy) in the Rabbit Kidney. J Urol. 2006;175:734–8. https://doi.org/10.1016/S0022-5347(05)00141-2.
Vlaisavljevich E, Kim Y, Owens G, et al. Effects of tissue mechanical properties on susceptibility to histotripsy-induced tissue damage. Phys Med Biol. 2013;59:253–70. https://doi.org/10.1088/0031-9155/59/2/253PMID-24351722.
Hall TL, Kieran K, Ives K, et al. Histotripsy of rabbit renal tissue in vivo: temporal histologic trends. J Endourol. 2007;21:1159–66. https://doi.org/10.1089/end.2007.9915.
Vlaisavljevich E, Maxwell A, Mancia L, et al. Visualizing the Histotripsy Process: Bubble Cloud-Cancer Cell Interactions in a Tissue-Mimicking Environment. Ultrasound Med Biol. 2016;42:2466–77. https://doi.org/10.1016/j.ultrasmedbio.2016.05.018.
Lake AM, Xu Z, Wilkinson JE, et al. Renal Ablation by Histotripsy—Does it Spare the Collecting System? J Urol. 2008;179:1150–4. https://doi.org/10.1016/j.juro.2007.10.033.
Roberts WW. Development and translation of histotripsy: current status and future directions. Curr Opin Urol. 2014;24:104–10. https://doi.org/10.1097/MOU.0000000000000001.
Kim Y, Vlaisavljevich E, Owens GE, et al. In vivo transcostal histotripsy therapy without aberration correction. Phys Med Biol. 2014;59:2553–68. https://doi.org/10.1088/0031-9155/59/11/2553.
Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 2017;27:138–48. https://doi.org/10.1007/s00330-016-4357-8.
ter Haar G. Acoustic Surgery. Phys Today. 2001;54:29–34. https://doi.org/10.1063/1.1445545.
Yu H, Burke CT. Comparison of Percutaneous Ablation Technologies in the Treatment of Malignant Liver Tumors. Semin Interv Radiol. 2014;31:129–37. https://doi.org/10.1055/s-0034-1373788.
Quesson B, Merle M, Köhler MO, et al. A method for MRI guidance of intercostal high intensity focused ultrasound ablation in the liver. Med Phys. 2010;37:2533–40. https://doi.org/10.1118/1.3413996.
Jung SE, Cho SH, Jang JH, Han J-Y. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdom Imaging. 2011;36:185–95. https://doi.org/10.1007/s00261-010-9628-2.
Fischer K, Gedroyc W, Jolesz FA. Focused Ultrasound as a Local Therapy for Liver Cancer. Cancer J. 2010;16:118–24. https://doi.org/10.1097/PPO.0b013e3181db7c32.
Wu F, Wang Z-B, Chen W-Z, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11:149–54. https://doi.org/10.1016/j.ultsonch.2004.01.011.
Macoskey JJ, Hall TL, Sukovich JR, et al. Soft-Tissue Aberration Correction for Histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2018;65:2073–85. https://doi.org/10.1109/TUFFC.2018.2872727.
Gerhardson T, Sukovich JR, Pandey AS, et al. Catheter Hydrophone Aberration Correction for Transcranial Histotripsy Treatment of Intracerebral Hemorrhage: Proof-of-Concept. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64:1684–97. https://doi.org/10.1109/TUFFC.2017.2748050.
Wang TY, Xu Z, Hall TL, et al. Active focal zone sharpening for high-precision treatment using histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:305–15. https://doi.org/10.1109/TUFFC.2011.1808.
Li J-J, Xu G-L, Gu M-F, et al. Complications of high intensity focused ultrasound in patients with recurrent and metastatic abdominal tumors. World J Gastroenterol. 2007;13:2747–51. https://doi.org/10.3748/WJG.V13.I19.2747.
Bader KB, Haworth KJ, Maxwell AD, Holland CK. Post Hoc Analysis of Passive Cavitation Imaging for Classification of Histotripsy-Induced Liquefaction in Vitro. IEEE Trans Med Imaging. 2018;37:106–15. https://doi.org/10.1109/TMI.2017.2735238.
Anthony GJ, Bollen V, Hendley S, et al. Assessment of histotripsy-induced liquefaction with diagnostic ultrasound and magnetic resonance imaging in vitro and ex vivo. Phys Med Biol. 2019;64. https://doi.org/10.1088/1361-6560/ab143f.
Hansen PD, Rogers S, Corless CL, et al. Radiofrequency ablation lesions in a pig liver model. J Surg Res. 1999. https://doi.org/10.1006/jsre.1999.5709.
Brace CL, Laeseke PF, Sampson LA, et al. Microwave ablation with a single small-gauge triaxial antenna: In vivo porcine liver model. Radiology. 2007;242:435–40. https://doi.org/10.1148/radiol.2422051411.
Acknowledgements
The authors of this study would like to acknowledge the HistoSonics R&D team (Josh Stopek PhD, Jon Cannata PhD, Ryan Miller PhD, and Alex Duryea PhD) and the animal anesthesia and care team.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study has received funding from HistoSonics, Inc. (Ann Arbor, MI). Author EV is a consultant, stockholder, and receives research support from HistoSonics, Inc. Author ZX is a stockholder and receives research support from HistoSonics, Inc. Author PL is a consultant and stockholder with HistoSonics, Inc., a consultant with NeuWave/Ethicon, Inc., and receives research support from Siemens Healthineers. Author TH is a consultant, stockholder, and receives research support from Histosonics, Inc. Author FL is a consultant, stockholder, receives research support, and is on the board of directors at HistoSonics, Inc., is a consultant with Ethicon, Inc., and has patents and royalties with Medtronic, Inc. Author TZ is a consultant, stockholder, receives research support from HistoSonics, Inc. and is a consultant with Ethicon, Inc.)
Informed consent
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. For this type of study, informed consent is not required. For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Knott, E.A., Longo, K.C., Vlaisavljevich, E. et al. Transcostal Histotripsy Ablation in an In Vivo Acute Hepatic Porcine Model. Cardiovasc Intervent Radiol 44, 1643–1650 (2021). https://doi.org/10.1007/s00270-021-02914-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-021-02914-1