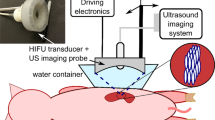
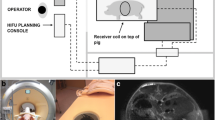
Abstract
Purpose
Robotically assisted sonic therapy (RAST) is a nonthermal, noninvasive ablation method based on histotripsy. Prior animal studies have demonstrated the ability to create hepatic ablation zones at the focal point of an ultrasound therapy transducer; however, these treatments resulted in thermal damage to the body wall within the path of ultrasound energy delivery. The purpose of this study was to evaluate the efficacy and safety of a pulse sequence intended to mitigate prefocal body wall injury.
Materials and Methods
Healthy swine (n = 6) underwent hepatic RAST (VortxRx software version 1.0.1.3, HistoSonics, Ann Arbor MI) in the right hepatic lobe. A 3.0 cm spherical ablation zone was prescribed for each. Following treatment, animals underwent MRI which was utilized for ablation zone measurement, evaluation of prefocal injury, and assessment of complications. Each animal was euthanized, underwent necropsy, and the tissue was processed for histopathologic analysis of the ablation zone and any other sites concerning for injury.
Results
No prefocal injury was identified by MRI or necropsy in the body wall or tissues overlying the liver. Ablation zones demonstrated uniform cell destruction, were nearly spherical (sphericity index = 0.988), and corresponded closely to the prescribed size (3.0 × 3.1 × 3.4 cm, p = 0.70, 0.36, and 0.01, respectively). Ablation zones were associated with portal vein (n = 3, one occlusive) and hepatic vein thrombosis (n = 4, one occlusive); however, bile ducts remained patent within ablation zones (n = 2).
Conclusions
Hepatic RAST performed with a modified ultrasound pulse sequence in a porcine model can mitigate prefocal body wall injuries while maintaining treatment efficacy. Further study of hepatic RAST appears warranted, particularly in tumor models.





Similar content being viewed by others
References
Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115–20. https://doi.org/10.1002/lt.20034.
Ziemlewicz TJ, Wells SA, Lubner MG, et al. Hepatic tumor ablation. Surg Clin North Am. 2016;96:315–39. https://doi.org/10.1016/j.suc.2015.12.006.
Lahat E, Eshkenazy R, Zendel A, et al. Complications after percutaneous ablation of liver tumors: a systematic review. Hepatobiliary Surg Nutr. 2014;3:317–23. https://doi.org/10.3978/j.issn.2304-3881.2014.09.07.
Livraghi T, Meloni F, Solbiati L, et al. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868–74. https://doi.org/10.1007/s00270-011-0241-8.
Velez E, Goldberg SN, Kumar G, et al. Hepatic thermal ablation: effect of device and heating parameters on local tissue reactions and distant tumor growth. Radiology. 2016;281:782–92. https://doi.org/10.1148/radiol.2016152241.
Poon RT, Ng KK, Lam CM, et al. Learning curve for radiofrequency ablation of liver tumors—prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441–9. https://doi.org/10.1097/01.sla.0000118565.21298.0a.
Hall TL, Hempel CR, Wojno K, et al. Histotripsy of the prostate: dose effects in a chronic canine model. Urology. 2009;74:932–7. https://doi.org/10.1016/j.urology.2009.03.049.
Parsons JE, Cain CA, Abrams GD, et al. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol. 2006;32:115–29. https://doi.org/10.1016/j.ultrasmedbio.2005.09.005.
Roberts WW, Hall TL, Ives K, et al. Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175:734–8. https://doi.org/10.1016/s0022-5347(05)00141-2.
Xu Z, Ludomirsky A, Eun LY, et al. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:726–36.
Vlaisavljevich E, Maxwell A, Mancia L, et al. Visualizing the histotripsy process: bubble cloud-cancer cell interactions in a tissue-mimicking environment. Ultrasound Med Biol. 2016;42:2466–77. https://doi.org/10.1016/j.ultrasmedbio.2016.05.018.
Vlaisavljevich E, Owens G, Lundt J, et al. Non-invasive liver ablation using histotripsy: preclinical safety study in an in vivo porcine model. Ultrasound Med Biol. 2017;43:1237–51. https://doi.org/10.1016/j.ultrasmedbio.2017.01.016.
Vlaisavljevich E, Kim Y, Allen S, et al. Image-guided non-invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model. Ultrasound Med Biol. 2013;39:1398–409. https://doi.org/10.1016/j.ultrasmedbio.2013.02.005.
Smolock AR, Cristescu MM, Vlaisavljevich E, et al. Robotically assisted sonic therapy as a noninvasive nonthermal ablation modality: proof of concept in a porcine liver model. Radiology. 2018. https://doi.org/10.1148/radiol.2018171544.
Wadell H. Volume, shape, and roundness of quartz particles. J Geol. 1935;43:250–80. https://doi.org/10.1086/624298.
Li JJ, Gu MF, Luo GY, et al. Complications of high intensity focused ultrasound for patients with hepatocellular carcinoma. Technol Cancer Res Treat. 2009;8:217–24. https://doi.org/10.1177/153303460900800306.
ter Haar G. High intensity ultrasound. Semin Laparosc Surg. 2001;8:77–89. https://doi.org/10.1177/155335060100800109.
Jung SE, Cho SH, Jang JH, et al. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdom Imaging. 2011;36:185–95. https://doi.org/10.1007/s00261-010-9628-2.
Khokhlova TD, Wang YN, Simon JC, et al. Ultrasound-guided tissue fractionation by high intensity focused ultrasound in an in vivo porcine liver model. Proc Natl Acad Sci USA. 2014;111:8161–6. https://doi.org/10.1073/pnas.1318355111.
Vlaisavljevich E, Kim Y, Owens G, et al. Effects of tissue mechanical properties on susceptibility to histotripsy-induced tissue damage. Phys Med Biol. 2014;59:253–70. https://doi.org/10.1088/0031-9155/59/2/253.
Harari CM, Magagna M, Bedoya M, et al. Microwave ablation: comparison of simultaneous and sequential activation of multiple antennas in liver model systems. Radiology. 2016;278:95–103. https://doi.org/10.1148/radiol.2015142151.
Yu NC, Raman SS, Kim YJ, et al. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19:1087–92. https://doi.org/10.1016/j.jvir.2008.03.023.
Styn NR, Wheat JC, Hall TL, et al. Histotripsy of VX-2 tumor implanted in a renal rabbit model. J Endourol. 2010;24:1145–50. https://doi.org/10.1089/end.2010.0123.
Lake AM, Hall TL, Kieran K, et al. Histotripsy: minimally invasive technology for prostatic tissue ablation in an in vivo canine model. Urology. 2008;72:682–6. https://doi.org/10.1016/j.urology.2008.01.037.
Hempel CR, Hall TL, Cain CA, et al. Histotripsy fractionation of prostate tissue: local effects and systemic response in a canine model. J Urol. 2011;185:1484–9. https://doi.org/10.1016/j.juro.2010.11.044.
Cornelis FH, Durack JC, Kimm SY, et al. A comparative study of ablation boundary sharpness after percutaneous radiofrequency, cryo-, microwave, and irreversible electroporation ablation in normal swine liver and kidneys. Cardiovasc Intervent Radiol. 2017;40:1600–8. https://doi.org/10.1007/s00270-017-1692-3.
Denys A, Lachenal Y, Duran R, et al. Use of high-frequency jet ventilation for percutaneous tumor ablation. Cardiovasc Intervent Radiol. 2014;37:140–6. https://doi.org/10.1007/s00270-013-0620-4.
Nykonenko A, Vavra P, Zonca P. Anatomic peculiarities of pig and human liver. Exp Clin Transplant. 2017;15:21–6.
Acknowledgements
The authors would like to acknowledge the HistoSonics R&D team, Jim Bertolina, Jon Cannata, Alex Duryea, Ryan Miller, and Zeljko Mladenovic for developing the pulse sequence evaluated in this study and for technical support during the study.
Funding
This study was funded by HistoSonics, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Fred T. Lee Jr., MD—Ethicon, Inc.: Consultant, HistoSonics, Inc.: Board Member, Advisor, Stockholder. Amanda Smolock MD, PhD—HistoSonics, Inc.: Advisor, Stockholder. Eli Vlaisavljevich, PhD—HistoSonics, Inc.: Advisor, Stockholder. Zhen Xu, PhD—HistoSonics, Inc.: Founder, Advisor, Stockholder. Timothy Ziemlewicz, MD—Ethicon, Inc.: Consultant, HistoSonics, Inc.: Advisor, Stockholder.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Approval was obtained from the Institutional animal use and care committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Longo, K.C., Knott, E.A., Watson, R.F. et al. Robotically Assisted Sonic Therapy (RAST) for Noninvasive Hepatic Ablation in a Porcine Model: Mitigation of Body Wall Damage with a Modified Pulse Sequence. Cardiovasc Intervent Radiol 42, 1016–1023 (2019). https://doi.org/10.1007/s00270-019-02215-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02215-8